Complete the following equations for nuclear reactions: 131 (a) B+? 2n + N (b) ? + Dn
Question:
Complete the following equations for nuclear reactions: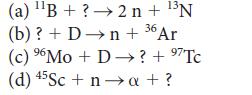
Transcribed Image Text:
131 (a) B+? 2n + N (b) ? + Dn + 36 Ar (c) Mo + D ? + 7Tc (d) 45Sc + na + ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
a B a2 n N 3...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The following equations are for nuclear reactions that are known to occur in the explosion of an atomic bomb. Identify X. 140 235 144
-
Complete the following nuclear reactions and find their Q values (use Appendix V for masses if necessary): (a) Li +_ (b) U + in (c) Be(a, %C He + He 5(dn) + Sr
-
Write balanced nuclear equations for the following reactions and identify X:
-
The Justice Department has been asked to review a merger request for a market with the following four firms. Firm Assets A .......... $156 million B .......... 130 million C .......... 45 million D...
-
Use the step-by-step method to find io(i) for t > 0 in the network in figure. 4 mA 100 F 4 kn 2 kn ww ww 2 kn 2 kn i.() 2 kn 12 kn +)4V 2 kn
-
Why would a company prefer gross revenue reporting over net revenue reporting?
-
What are the critical areas of yield management success?
-
Product T has revenue of $56,000, variable cost of goods sold of $40,000, variable selling expenses of $6,000, and fixed costs of $15,000, creating a loss from operations of $5,000. a. Determine the...
-
In your opinion what are the steps used by an auditor to evaluate an entity's ability to continue as a going concern ?
-
Deoxyglucose labeled with fluorine-18 is commonly used in PET scans to locate tumors. Fluorine-18 has a half-life of 109 min. How long will it take for the level of fluorine-18 in the body to drop to...
-
Actinium-225 decays by successive emission of three a particles. (a) Write the nuclear equations for the three decay processes. (b) Compare the neutron-to-proton ratio of the final daughter product...
-
Wheel Place Company began operations on March 1, 2019, to provide automotive wheel alignment and balancing services. On March 31, 2019, the unadjusted balances of the firm's accounts are as follows....
-
Daisy Cakes website on YouTube: https://www.youtube.com/watch?v=AVM-RuLh2KI Daisy Cakes is looking for financing from the sharks to expand her business. You will realize that Kim, the founder of...
-
Comparative financial statements for Weller Corporation, a merchandising company, for the year ending December 31 appear below. The company did not issue any new common stock during the year. A total...
-
Laker Company reported the following January purchases and sales data for its only product. For specific identification, ending inventory consists of 280 units from the January 30 purchase, 5 units...
-
Compensation (wages) Income taxes withheld $ 36,600 7,680 FICA taxes at a 7.65% rate (no employee had reached the maximum). Required: A. Prepare the March 31, 2022 journal entry to record the payroll...
-
Process Costing and Spoilage Nation Lovers PLC produces several items to be used as replacement tools for various types of machineries. The product costing system for NL which is used as spare part...
-
(a) dl-Glutamic acid has been synthesized from diethyl acetamidomalonate in the following way. Outline the reactions involved. (b) Compound G has also been used to prepare the amino acid dl-ornithine...
-
Determine which of the following limits exist. Compute the limits that exist. lim x-0 1- + 3x X
-
The rigid bar is pinned at A and supported by two aluminum rods, each having a diameter of 1 in., a modulus of elasticity E al = 10(10 3 ) ksi, and yield stress of (Ï Y ) al = 40 ksi. If the bar...
-
The specimen represents a filament-reinforced matrix system made from plastic (matrix) and glass (fiber). If there are n fibers, each having a cross-sectional area of A f and modulus of E f ,...
-
The support consists of a solid red brass C83400 copper post surrounded by a 304 stainless steel tube. Before the load is applied the gap between these two parts is 1 mm. Given the dimensions shown,...
-
exercise 4-7 (Algo) Effects of transactions on income statement LO P2
-
Compute the value of ordinary bonds under the following circumstances assuming that the coupon rate is 0.06:(either the correct formula(s) or the correct key strokes must be shown here to receive...
-
A tax-exempt municipal bond has a yield to maturity of 3.92%. An investor, who has a marginal tax rate of 40.00%, would prefer and an otherwise identical taxable corporate bond if it had a yield to...

Study smarter with the SolutionInn App


