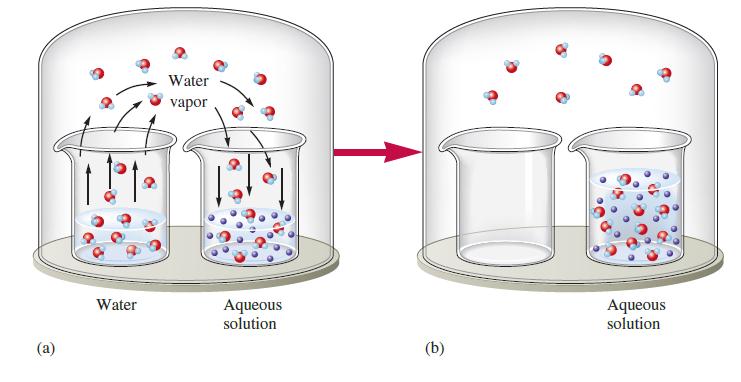
Consider Fig. 17.8. Suppose that instead of having a nonvolatile solute in the solvent in one beaker,
Question:
Consider Fig. 17.8. Suppose that instead of having a nonvolatile solute in the solvent in one beaker, the two beakers have different volatile liquids. That is, suppose one beaker contains liquid A (Pvap 5 50 torr) and the other beaker contains liquid B (Pvap 5 100 torr). Explain what happens as time passes. How is this similar to the first case shown in the figure? How is it different?
Fig. 17.8
Transcribed Image Text:
(a) Water Water vapor Aqueous solution (b) Aqueous solution
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
In Fig 178 if the two beakers contain different volatile liquids instead of a nonvolatile solute in ...View the full answer

Answered By

BillClinton Muguai
I have been a tutor for the past 5 years. I have experience working with students in a variety of subject areas, including computer science, math, science, English, and history. I have also worked with students of all ages, from elementary school to college. In addition to my tutoring experience, I have a degree in education from a top university. This has given me a strong foundation in child development and learning theories, which I use to inform my tutoring practices.
I am patient and adaptable, and I work to create a positive and supportive learning environment for my students. I believe that all students have the ability to succeed, and it is my job to help them find and develop their strengths. I am confident in my ability to tutor students and help them achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Suppose that instead of having a rights issue of new stock at 4, Pandora decided to make a general cash offer at 4. Would existing shareholders still be just as well off? Explain.
-
Suppose that instead of using 16 bits for the network part of a class B address originally, 20 bits had been used. How many class B networks would there have been?
-
Suppose that instead of swapping element A[i] with a random element from the subarray A[i ..n], we swapped it with a random element from anywhere in the array: PERMUTE-WITH-ALL (A) 1 n length [A] 2...
-
Read Exhibit 10.6 carefully and answer the following question: Can a free-market system be trusted to effectively address the global concern in biodiversity loss? Why or why not? Exhibit 10.6 EXHIBIT...
-
The monthly utility bills in a city are normally distributed, with a mean of $100 and a standard deviation of $12. Find the probability that a randomly selected utility bill is (a) Less than $70, (b)...
-
Three $10 000, 10.5% bonds with quarterly coupons redeemable at par on August 1, 2025, were bought on May 1, 2011, to yield 12% compounded quarterly. The bonds were sold on January 16, 2019, at 93.5.
-
Calculate SS, variance, and standard deviation for the following population of N 5 8 scores: 1, 3, 1, 10, 1, 0, 1, 3. (Note: The computational formula works well with these scores.)
-
A tennis enthusiast wants to estimate the mean length of mens singles matches held during the Wimbledon tennis tournament. From the Wimbledon history archives, he randomly selects 40 matches played...
-
The following bond list is from the business section of a financial newspaper on January 1, 2016. Assume that each bond shown matures on January 1 in 5, 10, or 30 years. Each bond shown pays a...
-
The ages of 20 dogs in a pet shelter are shown. Construct a frequency distribution using 7 classes. 3 6. 4 4 9. 4 3 4 9.
-
Without referring to your text, predict the trend of second ionization energies for the elements sodium through argon. Compare your answer with the data in Table 12.6. Explain any differences. Table...
-
Consider a Poisson probability distribution with = 8.5. Determine the mean and standard deviation of this distribution.
-
A PAM telemetry system involves the multiplexing of four input signals: s i (t), i = 1, 2 3, 4. Two of the signals s 1 (t) and s 2 (t) have bandwidths of 80 Hz each, whereas the remaining two signals...
-
Exercise 11-5 Profit allocation in a partnership LO3 Dallas and Weiss formed a partnership to manage rental properties, by investing $198,000 and $242,000, respectively. During its first year, the...
-
Reading following articles and answer the questions: https://www.afr.com/technology/ai-is-coming-for-white-collar-jobs-gates-warns-20230123-p5cev7...
-
1. Citing an example in each case, briefly explain four types of book keeping errors which are not disclosed by trial balance 2. The trial balance extracted from the books of james as at 30 september...
-
Use the universal gravitation formula to determine which object has a larger effect on the Earth's motion through space: the Sun or the Moon. Explain how you are determining this, including very...
-
Pro Cycling Shop is a medium-size seller of the high-end bicycle. Since starting the company 15 years ago, Pro Cycling Shop has been a competitive company across Sarawak, Brunei, Kalimantan, and...
-
Solve each equation or inequality. |8 - 5x| 2
-
In Problems 718, write the augmented matrix of the given system of equations. f0.01x0.03y = 0.06 [0.13x + 0.10y = 0.20
-
As the pressure is increased at 45C, ice I is converted to ice II. Which of these phases has the lower density?
-
Compound A has molecular formula C 5 H 10 . Hydroboration-oxidation of compound A produces an alcohol with no chirality centers. Draw two possible structures for compound A.
-
A perfectly insulating box is partially filled with water in which an electrical resistor is immersed. An external electrical generator converts the change in potential energy of a mass m which falls...
-
Just work out the assignment on your own sheet, you dont need the excel worksheet. Classic Coffee Company Best friends, Nathan and Cody, decided to start their own business which would bring great...
-
Financial information related to the proprietorship of Ebony Interiors for February and March 2019 is as follows: February 29, 2019 March 31, 2019 Accounts payable $310,000 $400,000 Accounts...
-
(b) The directors of Maureen Company are considering two mutually exclusive investment projects. Both projects concern the purchase of a new plant. The following data are available for each project...

Study smarter with the SolutionInn App


