Without referring to your text, predict the trend of second ionization energies for the elements sodium through
Question:
Without referring to your text, predict the trend of second ionization energies for the elements sodium through argon. Compare your answer with the data in Table 12.6. Explain any differences.
Table 12.6
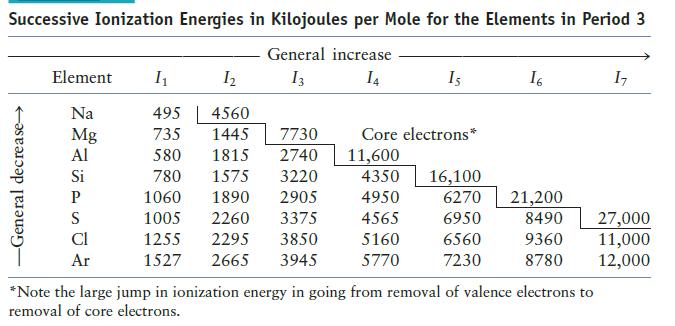
Transcribed Image Text:
Successive Ionization Energies in Kilojoules per Mole for the Elements in Period 3 General increase 13 14 -General decrease Element I₁ Na Mg Al Si P S Cl Ar 1₂ 4560 1445 7730 1815 2740 780 1575 3220 1060 1890 2905 1005 2260 3375 1255 2295 3850 1527 2665 3945 495 735 580 Is Core electrons* 11,600 4350 4950 4565 5160 5770 16,100 6270 6950 6560 7230 16 17 21,200 8490 27,000 9360 11,000 8780 12,000 *Note the large jump in ionization energy in going from removal of valence electrons to removal of core electrons.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
Without referring to the text I predict that the second ionization energies for the elements sodium ...View the full answer

Answered By

FELIX NYAMBWOGI
I have been tutoring for over 5 years, both in person and online. I have experience tutoring a wide range of subjects, including math, science, English, and history. I have also worked with students of all ages, from elementary school to high school.
In addition, I have received training in effective tutoring strategies and techniques, such as active listening, questioning, and feedback. I am also proficient in using online tutoring platforms, such as Zoom and Google Classroom, to effectively deliver virtual lessons.
Overall, my hands-on experience and proficiency as a tutor has allowed me to effectively support and guide students in achieving their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use Bonferroni t tests with the data in Table 18.1 to compare performance at the following points: (a) Week 0 and Week 6 (b) Week 0 and Week 12 (c) Week 3 and Week 12
-
Elements with very large ionization energies also tend to have highly exothermic electron affinities. Explain. Which group of elements would you expect to be an exception to this statement?
-
The ionization energies of sodium (in kJ/mol), starting with the first and ending with the eleventh, are 495.9, 4560, 6900, 9540, 13,400, 16,600, 20,120, 25,490, 28,930, 141,360, 170,000. Plot the...
-
Silverago Incorporated, an international metals company, reported a loss on the sale of equipment of $2 million in 2010. In addition, the companys income statement shows depreciation expense of $8...
-
The amounts of time per workout an athlete uses a stairclimber are normally distributed, with a mean of 20 minutes and a standard deviation of 5 minutes. Find the probability that a randomly selected...
-
Pharma Manufacturing is considering a medical process that is expected to reduce its annual operating costs by $60 000.00. What is the maximum amount of money that should be invested for the process...
-
Calculate SS, variance, and standard deviation for the following population of N 5 7 scores: 8, 1, 4, 3, 5, 3, 4. (Note: The definitional formula works well with these scores.)
-
You have the following information for Goodspeed Diamonds. Goodspeed Diamonds uses the periodic method of accounting for its inventory transactions. Goodspeed only carries one brand and size of...
-
CORPORATE FINANCE Use the future value interest factor to calculate the following questions: a. Assume that you keep $5,555 in the savings account at ABC Bank with an interest rate of 15 per cent per...
-
The file P02_35.xlsx contains data from a survey of 500 randomly selected households. For this problem, consider this data set a simple random sample from all possible households, where the number of...
-
Choose the best response for the following. The ionization energy for the chlorine atom is equal in magnitude to the electron affinity for : a. The Cl atom b. The Cl - ion c. The Cl+ ion d. The F...
-
Consider Fig. 17.8. Suppose that instead of having a nonvolatile solute in the solvent in one beaker, the two beakers have different volatile liquids. That is, suppose one beaker contains liquid A...
-
Select an organization that you think should reposition itself in the consumers eye. Identify where it is currently positioned, and make recommendations for repositioning. Explain and defend your...
-
Star Trek LLC has 8,000 bonds, two million shares of preferred stock outstanding and seven million shares of common stock outstanding. If the common shares are selling for $17 per share, the...
-
So the component of the flow velocity that is perpendicular to the isobar in cm/s is: V =2V cos(0)=2(10/(1+2 sin(0))) cos(0) V (10)/[1+2(1) sin(90)] = V = 3.3cm/s
-
2. The CIBC stock price was very volatile today. a. Using the website below, what did the price of CIBC shares end the day at ? CM.TO: Canadian Imperial Bank of Commerce - Yahoo Finance b. In a brief...
-
Answer the following according to Florida rules: Abigail Atlas was chatting quietly in the hall outside Courtroom 14-1 with Mariel Topher, an employee of the Hopper Law Firm that was representing...
-
Draw Free body diagrams for the following 32 situations and write the appropriate x- and y- equations, using the diagram and title to help you (do not solve). Some of the material you have not...
-
Solve each equation or inequality. |7x - 3| > 4
-
In Problems 1522, find the principal needed now to get each amount; that is, find the present value. To get $750 after 2 years at 2.5% compounded quarterly.
-
True or false? The atom with the largest subscript in a formula is the atom with the largest percent by mass in the compound. If true, explain why with an example. If false, explain why with an...
-
What information do we get from a chemical formula? From a chemical equation?
-
Atomic masses are relative masses. What does this mean?
-
Suppose the S&P 500 currently has a level of 960. One contract of S&P 500 index futures has a size of $250 S&P 500 index. You wish to hedge an $800,000-portfolio that has a beta of 1.2. (A)In order...
-
Exhibit 4.1 The balance sheet and income statement shown below are for Koski Inc. Note that the firm has no amortization charges, it does not lease any assets, none of its debt must be retired during...
-
Haley is 57 years of age. She is planning for future long-term care needs. She knows that yearly nursing home costs in her area are currently $69,000, with prices increased by 5 percent annually....

Study smarter with the SolutionInn App


