Consider the following data concerning four different substances. Label the four substances as either ionic, network, metallic,
Question:
Consider the following data concerning four different substances.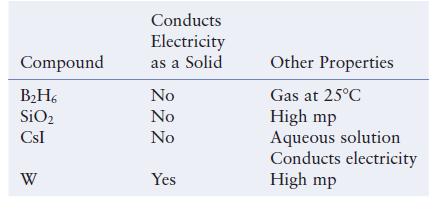
Label the four substances as either ionic, network, metallic, or molecular solids.
Transcribed Image Text:
Compound B₂H6 SiO₂ CsI W Conducts Electricity as a Solid No No No Yes Other Properties Gas at 25°C High mp Aqueous solution Conducts electricity High mp
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
B2H6 Ionic SiO2 Network CsI Ionic W Metallic Explanation B2H6 is an ionic ...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the following data concerning four different substances. Label the four substances as either ionic, network, metallic, or molecular solids. Conducts Electricity as a Solid Othe Properties No...
-
Part 1 a. Ammonia, NH 3 , is a weak electrolyte. It forms ions in solution by reacting with water molecules to form the ammonium ion and hydroxide ion. Write the balanced chemical reaction for this...
-
Consider the following data concerning the demand (y) and price (x) of a consumer product. a. Plot y versus x. Does it seem reasonable to use the simple linear regression model to relate y to x? b....
-
With respect to strategies used by land conservation groups to preserve land, conservation easements seem to be expanding more rapidly than buying land for preservation. In what respect might...
-
The scores of three students in a study group on a test are 98, 95, and 93. Use a sample size of 3. Find the mean and standard deviation of the population. List all samples (with replacement) of the...
-
Express each of the following as a percent. 11/40
-
Dem bones Archaeopteryx is an extinct beast having feathers like a bird but teeth and a long bony tail like a reptile. Only six fossil specimens are known. Because these specimens differ greatly in...
-
On the basis of the following data, determine the value of the inventory at the lower of cost or market. Assemble the data in the form illustrated in Exhibit8. Inventory Unit Unit Cost Price $ 80...
-
4 Here are book- and market value balance sheets of the United Frypan Company (figures in $ millions): $ 3.5 points Book-Value Balance Sheet Net working capital $ 50 Debt Long-term assets 50 Equity $...
-
Suppose that you are fixed payer on a $80M-notional plain-vanilla 4-year interest-rate swap with rate c=3%. In additional you have a short 2-year cap position on $65M notional with rate K C =4.2% and...
-
Use the diagram of the unit cell for the hexagonal closest packed structure in Fig. 16.14 to determine the net number of atoms in the hcp unit cell. Fig. 16.14 b a b 5 10 hep 12 3 Figure 16.16 The...
-
How could you tell experimentally if TiO 2 is an ionic solid or a network solid?
-
Should you use Method or Methodology to describe how the research was conducted? Why?
-
Leslie Sporting Goods is a locally owned store that specializes in printing team jerseys. The majority of its business comes from orders for various local teams and organizations. While Leslie's...
-
Euclid acquires a 7-year class asset on May 9, 2022, for $153,000 (the only asset acquired during the year). Euclid does not elect immediate expensing under 179. He does not claim any available...
-
Williams & Sons last year reported sales of $10 million, cost of goods sold (COGS) of $8 million, and an inventory turnover ratio of 2. The company is now adopting a new inventory system. If the new...
-
A ceramic manufacturer promised to deliver 25 crates of vases to a Japanese importer under a "CFR" INTERCOM agreement. During transit, however, a large number of vases were broken. The buyer wants to...
-
A company receives $364, of which $23 is for sales tax. The journal entry to record the sale would include a ?
-
Fill in the blank(s) to correctly complete each sentence. The graph of (x) = (x + 4) 2 is obtained by shifting the graph of y = x 2 to the________ 4 units.
-
In Exercises discuss the continuity of each function. f(x) -3 1 x - 4 y 3 2 -1 -2 -3+ 3 X
-
Give an example based on moleculemolecule interactions excluding chemical reactions, illustrating how the total pressure upon mixing two real gases could be different from the sum of the partial...
-
Draw the expected major product for each of the following reactions: a. b. c. d. e. f. ? HBr
-
A triple point refers to a point in a PT phase diagram for which three phases are in equilibrium. Do all triple points correspond to a gasliquidsolid equilibrium?
-
Lou Barlow, a divisional manager for Sage Company, has an opportunity to manufacture and sell one of two new products for a five - year period. His annual pay raises are determined by his division s...
-
Consider a 5 year debt with a 15% coupon rate paid semi-annually, redeemable at Php1,000 par. The bond is selling at 90%. The flotation cost is Php50 per bind. The firm's tax bracket is 30%.
-
A project will generate annual cash flows of $237,600 for each of the next three years, and a cash flow of $274,800 during the fourth year. The initial cost of the project is $749,600. What is the...

Study smarter with the SolutionInn App


