Consider the following galvanic cell: What happens to (mathscr{E}) as the concentration of (mathrm{Zn}^{2+}) is increased? as
Question:
Consider the following galvanic cell:
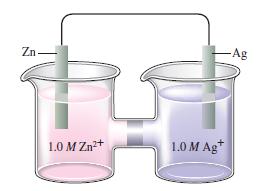
What happens to \(\mathscr{E}\) as the concentration of \(\mathrm{Zn}^{2+}\) is increased? as the concentration of \(\mathrm{Ag}^{+}\)is increased? What happens to \(\mathscr{E}^{\circ}\) in these cases?
Transcribed Image Text:
Zn 1.0 M Zn+ 1.0 M Ag+ -Ag
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
In order to examine the variations in both standard cell potential C and cell potential E E We may u...View the full answer

Answered By

Akash Goel
I am in the teaching field since 2008 when i was enrolled myself in chartered accountants course
Since then i have an experience of teaching of class XI, XII, BCOM, MCOM, MBA, CA CPT.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the following galvanic cells: For each galvanic cell, give the balanced cell equation and determine Ïo. Standard reduction potentials are found in Table 11.1. Table 11.1 1.0 M Cut 1.0 M...
-
Consider the following galvanic cell at 25oC: Pt | Cr2+ (0.30 M), Cr3+(2.0 M) | | Co2+(0.20 M) | Co The overall reaction and equilibrium constant value are 2Cr2+(aq) + Co2+(aq) 2Cr3+(aq) + Co(s) K =...
-
Consider the following galvanic cell: Calculate the concentrations of Ag+(aq) and Ni2+(aq) once the cell is dead. Ni 1.0 MNi2+ 10 M Ag
-
Should U.S. national forests become privatized (sold to private owners)? Why or why not?
-
Include air resistance proportional to the square of the balls speed in the previous problem. Let the drag coefficient be cw = 0.5, the softball radius be 5 cm and the mass be 200 g. (a) Find the...
-
In each of the following situations, discuss how Hippo Vaughn might use stock index futures to protect a well-diversified stock portfolio: a. Hippo expects to receive a sizable bonus check next month...
-
Have they begun to think about these in the organisations you have studied? Collect new examples of one operational and one management information system, from someone working in an organisation.
-
Lauren Moore, a professor in management science, is computing her final grades for her introductory management science class. The average final grade is a 63, with a standard deviation of 10....
-
Here is an information regarding the relevant PDC company policies The beginning cash balance is $23.000. The building rent is fixed at a monthly rate of $ 4,600. In the period January - June 2019...
-
The saturated calomel electrode, abbreviated SCE, is often used as a reference electrode in making electrochemical measurements. The SCE is composed of mercury in contact with a saturated solution of...
-
What is the difference between \(\mathscr{E}\) and \(\mathscr{E}^{\circ}\) ? When is \(\mathscr{E}\) equal to zero? When is \(\mathscr{E}^{\circ}\) equal to zero? (Consider "regular" galvanic cells...
-
A standard procedure for improving the detection of the stoichiometric point in titrations of weak bases with strong acids is to use acetic acid as a solvent. Explain the basis of this approach.
-
Avery, an unmarried taxpayer, had the following income items: Salary Net income from a rental house 3 7 , 0 5 0 4 , 9 0 0 Avery has a 4 - year - old child who attends a child care center. Assume the...
-
California Lottery Let A denote the event of placing a $1 straight bet on the California Daily 4 lottery and winning. There are 10,000 different ways that you can select the four digits (with...
-
"Tamara Wiley glanced in the mirror before leaving her apartment and heading to her 8 a.m. class. She was having a bad hair day, so she had thrown on a scarf. Her quick check in the mirror told her...
-
Online Friends In a Pew Research Center survey of 1060 teens aged 13 to 17, it was found that 604 (or 57.0%) of those respondents have made new friends online. If the true rate is 50%, there is a...
-
Dr. Yong has requested that Senture Houston, an office manager at Pain Free Dental Associates, prepare a single journal entry for December 31, 2022. The bank statement for that day shows $9,500....
-
Show that the thermal efficiency of a combined gas-steam power plant ï¨cc can be expressed as where ï¨g = Wg /Qin and ï¨s = Ws /Qg,out are the thermal efficiencies of the...
-
What can you do to reduce hunger where you live? To reduce hunger globally?
-
Calculate the equilibrium constants for the following reactions: (a) Mn(s) + Ti+ (aq) = (b) In+ (aq) + U+ (aq) U+ (aq) Mn+ (aq) + Ti(s) In+ (aq) + U4+ (aq)
-
You are an analytical chemist and are working out a procedure for precipitating lead from a solution of lead nitrate. You know that lead(II) iodide is insoluble, so you decide to use potassium iodide...
-
For reactions in which the equilibrium constant is very large or very small, it can be difficult to measure the concentration of all species in solution in order to determine K. An alternative method...
-
Which of the following statements regarding traditional cost accounting systems is false? a. Products are often over or under cost in traditional cost accounting systems. b. Most traditional cost...
-
Bart is a college student. Since his plan is to get a job immediately after graduation, he determines that he will need about $250,000 in life insurance to provide for his future wife and children...
-
Reporting Financial Statement Effects of Bond Transactions (please show me how you got the answers) Lundholm, Inc., which reports financial statements each December 31, is authorized to issue...

Study smarter with the SolutionInn App


