Consider the following illustrations: Which beaker best illustrates what happens when the following acids are dissolved in
Question:
Consider the following illustrations: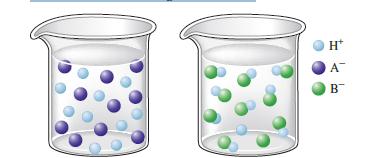
Which beaker best illustrates what happens when the following acids are dissolved in water?
a. HNO2
b. HNO3 e. HC2H3O2
c. HCl
d. HF
e. HC2H3O2
Transcribed Image Text:
H* A B
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (8 reviews)
d HF The beaker on the right with the blue liquid shows what happens when HF ...View the full answer

Answered By

Hande Dereli
Enthusiastic tutor, skilled in ACT and SAT tutoring. Raised one student's score on the SATs from 1100 combined to 1400. Graduated with a 3.9 GPA from Davidson College and led a popular peer tutoring group for three years. Scored in the top 0.06% in the nation on the SATs. The real reason I'm the one to help you nail the test? Results. Clients invariably praise my ability to listen and communicate in a low-stress, fun way. I think it's that great interaction that lets me raise retest SAT scores an average of 300 points.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the following illustrations:
-
Consider the following illustrations: Which beaker best illustrates what happens when the following acids are dissolved in water? a. HNO 2 b. HNO 3 c. HCl d. HF e. HC 2 H 3 O 2 H+ A B
-
The purpose of this problem is to get you used to the concept of autocorrelation in a time series. You could do this with any time series, but here you should use the series of Walmart daily stock...
-
3. Questions A venture capitalist (VC) is willing to invest 100m for 20% ownership of a start-up that is looking to achieve scale. All existing shares are common shares, and this deal would result in...
-
Suppose that the random variable X has the following p.d.f.: Construct a random variable Y = r(X) that has the uniform distribution on the interval [0, 5]. 2e-2 for> o, f(x)-| 2e- for x > 0. 0...
-
The disaccharide at the terminus of the oligosaccharide attached to the surface of type B red blood cells is an -D-galactopyranosyl--D-galactopyranose. The linkage is an acetal from C1 of the first...
-
Isabella provides 30 percent of the support for her father Hastings, who lives in an apartment by himself and has no gross income. Is it possible for Isabella to claim her father as a dependent?...
-
Engineering design is an activity that is vital to the success of any motor vehicle manufacturer. Identify the level at which engineering design would be classified in the cost hierarchy used with...
-
9. Using the ABC system, how much total manufacturing overhead cost is assigned to Product Y? Note: Round all intermediate calculations to 2 decimal places. 10. Using the ABC system, how much total...
-
View at least three examples of public speaking. You can investigate TV network news broadcasts, C-SPAN, or any other source. Describe each speakers gestures, expressions, voice levels, inflections,...
-
Is the conjugate base of a weak acid a strong base? Explain. Explain why Cl - does not affect the pH of an aqueous solution
-
You have two solutions of the salts NaX(aq) and NaY(aq) at equal concentrations. What would you need to know to determine which solution has the higher pH? Explain how you would decide (perhaps even...
-
Over what range of states are the various coefficients in Section 12.5 most useful?
-
C 2 H 6 O 2 + NaOH + 6 H 2 O C 2 H 3 NaO 3 + O 2 + 3 H 2Hydrogen is produced at the cathode, oxYGEN AT THE ANODE .Mass balance to produce 5000 tonnes a year of glycolic acid, formic acid and oxalic...
-
Please answer: a discussion of the ethical issues involved. The court might not itself consider the ethics of the actions of the parties. However, I ask that you consider the ethics of the following:...
-
In Exercises 21-24, use these results from the "1-Panel-THC" test for marijuana use, which is provided by the company Drug Test Success: Among 143 subjects with positive test results, there are 24...
-
I need help for an assignment of a review on research on Virtual Education on study motivation and academic performance in university students. I am attaching a research article from a magazine to...
-
Shouldice Hospital in Canada is widely known for one thing-hernia repair! In fact, that is the only operation it performs, and it performs a great many of them. Over the past two decades this small...
-
Rank in order of increasing average density: (a) Jupiter, (b) Saturn, (c) Earth
-
Integration is a vital concept when applied in one?s life. Integrating your life means making ideal choices. Perfect choices on the other go in line with quality decisions. Quality decisions lead to...
-
The base sequences in mRNA that code for certain amino acids are Glu: GAA, GAG Val: GUU, GUC, GUA, GUG Met: AUG Trp: UGG Phe: UUU,UUC Asp: GAU, GAC These sequences are complementary to the sequences...
-
The change of a single base in the DNA sequence for normal hemoglobin can encode for the abnormal hemoglobin, giving rise to sickle cell anemia. Which base in the codon for glu in DNA is replaced to...
-
The average molar mass of one base pair of nucleotides in DNA is approximately 600 g/mol. The spacing between successive base pairs is about 0.34 nm, and a complete turn in the helical structure of...
-
Logistics Solutions provides order fulfillment services for dot.com merchants. The company maintains warehouses that stock items carried by its dot.com clients. When a client receives an order from a...
-
Ohno Company specializes in manufacturing a unique model of bicycle helmet. The model is well accepted by consumers, and the company has enough orders to keep the factory production at 10,000 helmets...
-
Entries for Sale of Fixed Asset Equipment acquired on January 5 at a cost of $134,640, has an estimated useful life of 17 years, has an estimated residual value of $9,350, and is depreciated by the...

Study smarter with the SolutionInn App


