Consider the following illustrations: Which beaker best illustrates what happens when the following acids are dissolved in
Question:
Consider the following illustrations:
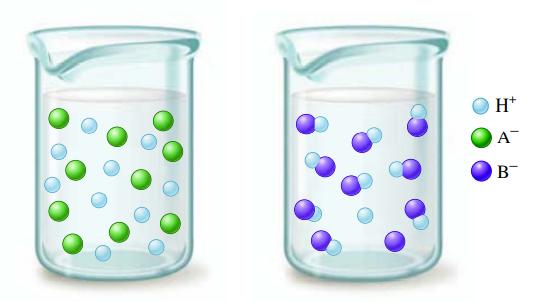
Which beaker best illustrates what happens when the following acids are dissolved in water?
a. HNO2
b. HNO3
c. HCl
d. HF
e. HC2H3O2
Transcribed Image Text:
H+ A B
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Consider the following illustrations:
-
Consider the following illustrations: Which beaker best illustrates what happens when the following acids are dissolved in water? a. HNO 2 b. HNO 3 e. HC 2 H 3 O 2 c. HCl d. HF e. HC 2 H 3 O 2 H* A B
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
With a global economy, the only way to achieve cost-effective control of greenhouse gases is to assure that every country imposes the same universal set of emissions standards. Discuss.
-
Does actual cash value equal fair market value?
-
1. Do you think that Hello Kitty will continue to rule the world? What are the pros and cons? 2. What are the reasons that Hello Kitty is licensed to so many different product manufacturers? 3....
-
What are some new features of Project 2010, and how do they differ from previous versions of Microsoft Project? LO.1
-
Main Street Service Co. has achieved fast growth in the St. Louis area by selling service contracts on large appliances, such as washers, dryers, and refrigerators. For a fee, Main Street agrees to...
-
Beta is an accepted measure of systematic risk. From the perspective of the investment analyst, discuss the uses and limitations of Beta and how it is applied as a tool for risk measurement.
-
Maryssa McFadden opened a public relations firm called Dance Fever on August 1, 2024. The following amounts summarize her business on August 31, 2024: (Click the icon to view the amounts.) Bal. a The...
-
What are the major species present in 0.250 M solutions of each of the following acids? Calculate the pH of each of these solutions. a. HClO 4 b. HNO 3
-
Calculate the concentration of an aqueous HBr solution that has pH = 4.25. HBr is a strong acid.
-
What are nominal, real and personal accounts? Give four examples of each of them.
-
how could playing in a sandbox help to the development of children? how could a garden help to the development of children? how could playground obstacle courses like a pebble bridge and monkey bars...
-
A store order bottles of shampoo throughout the year. Over time, the store has learned that the annual demand D for shampoo is constant, i.e., there is no variability. Currently, the store decides to...
-
Solve the Practice #2 == where L2 =02A = a, L404B = c, L = 0204 = d, y = /2 1) Find the velocity 3 when 82 = /2 and 6 = 0.4 rad/s 2) Find the acceleration 63 when = /2 and 62 = 0.4 rad/s 03. 03 Y B...
-
.0.5 0.5 For the above plot of the ellipsoid (22) 2- + +() + (-) = 1, find the parameters a, b and c. Note that a, b and c are positive integers between 1 and 6 inclusive. Use the mouse to rotate the...
-
The annual energy consumption of the University of Maryland is 100 million kWh. How much Uranium-235 is needed to produce this amount of energy in a nuclear power plant assuming 100% efficiency? (The...
-
Two sides and an angle are given. Determine whether the given information results in one triangle, two triangles, or no triangle at all. Solve any triangle(s) that results. a = 3, b = 7, A = 70
-
Choose a company from the SEC EDGAR Web site for your Key Assignment to evaluate for the impact of convergence to IFRS. Review the financial reports and notes of the company you have chosen from the...
-
Rank the following sets of substituents in order of Cahn-In-gold Prelog priorities: (a) CH3, 0H, H, C1 (b) CH3, CH2CH3, CH = CH2, CH2OH (c) CO2H, CH2OH, C = N, CH2NH2 (d) CH2CH3, C = CH, C = N,...
-
Assign F or Z configuration to the following alkenes: (a) CH2 (b) CCH C=C C=C CH3CH2 CI CH2CH2CH3 CH30 (e) CH (d) CN O C=C C=C CH2NH2 C-
-
Assign stereochemistry (E or Z) to the double bond in the following compound, and convert the drawing into a skeletal structure (red0):
-
Based on the regression output (below), would you purchase this actively managed fund with a fee of 45bps ? Answer yes or no and one sentence to explain why.
-
What is the yield to maturity on a 10-year, 9% annual coupon, $1,000 par value bond that sells for $967.00? That sells for $1,206.10?
-
1)Prepare the journal entry to record Tamas Companys issuance of 6,500 shares of $100 par value, 9% cumulative preferred stock for $105 cash per share. 2. Assuming the facts in part 1, if Tamas...

Study smarter with the SolutionInn App


