Consider the nitration of the compound If the reaction can be controlled so that one NO 2
Question:
Consider the nitration of the compound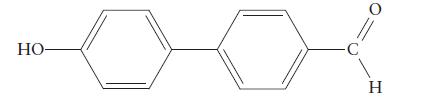 If the reaction can be controlled so that one NO2 group replaces one H atom of the molecule, where do you expect the nitro group to end up in the product?
If the reaction can be controlled so that one NO2 group replaces one H atom of the molecule, where do you expect the nitro group to end up in the product?
Transcribed Image Text:
H 0 HO-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The image shows a compound with two benzene rings connected by a single bond with one ring having a ...View the full answer

Answered By

Saud Ur Rehman
Evaluating manufacturing processes by designing and conducting research programs; applying knowledge of product design, fabrication, assembly, tooling, and materials; conferring with equipment vendors; soliciting observations from operators. Developing manufacturing processes by studying product requirements; researching, designing, modifying, and testing manufacturing methods and equipment; conferring with equipment vendors. Keeping equipment operational by coordinating maintenance and repair services; following manufacturer's instructions and established procedures; requesting special service.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The formal study of probability began with questions regarding gambling and games of chance. The conventional analysis of gambling is based on the expected values of these games which is always...
-
The presence of additional nitro groups can have an impact on the temperature at which a nucleophilic aromatic substitution will readily occur. Consider the following example. When both R groups are...
-
Consider Figs. 16.3 and 16.4 illustrating cascade control. (a) Suppose you were to apply feedforward control, instead of cascade control, to handle disturbances D 1 and D 2 . Where do you expect...
-
Suppose the comparative balance sheets of Sage Hill Inc.. are presented here. SAGE HILL INC. Condensed Balance Sheet May 31 ($ in millions) 2019 2018 Assets Current Assets $9,680 $8,760 Property,...
-
Using loop analysis and MATLAB, find Io in the network infigure. 12/0v 2/0 A j1a 4/0 A 320 -j2 0
-
Compare formal and informal channels of communication within organizations. Which is more valuable to employees?
-
8. When you purchase a new piece of electronic equipment, such as a computer or smartphone, do you read the instructions about how to use it? Answer each of the following questions with a Yes or No.
-
Assume that interest rate parity holds. In both the spot market and the 90-day forward market, 1 Japanese yen equals 0.0086 dollar. In Japan, 90-day risk-free securities yield 4.6%. What is the yield...
-
Required: 1. For HTCs hardware product revenue stream, what is the distinguishing factor that should be considered when determining whether HTC is acting as a principal or as an agent?
-
Identify the type and number of bonds on carbon atom 2 in (a) Pentane; (b) 2-pentene; (c) 2-pentyne.
-
Give the systematic name of (a) CH 3 COOH; (b) CH 3 CH 2 CH 2 COOH; (c) CH 2 (NH 2 )COOH.
-
A division of Raytheon owns a 5-year-old turret lathe used to manufacture fabricated metal products that was purchased for \($96,000\) and now has a financial reporting (non-tax) book value of...
-
Given the following memory status below, compute how much does it cost to compact holes together with the following compaction strategies if 1 kbyte of movement costs 50 centavos. 0. OS OS OS OS OS...
-
ITG Pte Ltd ("ITG") is a company specialising in air-conditioner maintenance and servicing. It makes adjusting and closing entries every 31 December, which is the company's financial year-end. Unless...
-
(20 points) We know that when we have a graph with negative edge costs, Dijkstra's algo rithm is not guaranteed to work. (a) Does Dijkstra's algorithm ever work when some of the edge costs are...
-
Create a new user called cis605_usr. Use Master. assign a password of abcd, set check_policy to off and check_expiration to off (Why set these two to off?). Execute the sp_addsrvrolemember to add the...
-
Salmone Company reported the following purchases and sales of its only product. Salmone uses a periodic inventory system. Determine the cost assigned to the ending inventory using FIFO. Date Units...
-
Repeat Problem 4 - 6 but use the values 50, 100, and 150 for the standard deviation. Superimpose all three curves on a single graph.
-
A sample statistic will not change from sample to sample. Determine whether the statement is true or false. If it is false, rewrite it as a true statement.
-
Draw a bondline structure showing the zwitterionic form of each of the following amino acids: (a) l-Valine (b) l-Tryptophan (c) l-Glutamine (d) l-Proline
-
The 20 naturally occurring amino acids (See the following table) are all l amino acids, and they all have the S configuration, with the exception of glycine (which lacks a chirality center) and...
-
Draw a Fischer projection for each of the following amino acids: (a) l-Threonine (b) l-Serine (c) l-Phenylalanine (d) l-Asparagine
-
Based on the regression output (below), would you purchase this actively managed fund with a fee of 45bps ? Answer yes or no and one sentence to explain why.
-
What is the yield to maturity on a 10-year, 9% annual coupon, $1,000 par value bond that sells for $967.00? That sells for $1,206.10?
-
1)Prepare the journal entry to record Tamas Companys issuance of 6,500 shares of $100 par value, 9% cumulative preferred stock for $105 cash per share. 2. Assuming the facts in part 1, if Tamas...

Study smarter with the SolutionInn App


