Determine whether each of the following electron configurations represents the ground state or an excited state of
Question:
Determine whether each of the following electron configurations represents the ground state or an excited state of the atom given.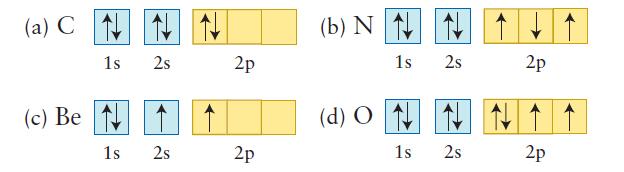
Transcribed Image Text:
(a) C (c) Be 1s 2s 1s 2s 2p 2p (b) N 1s 2s (d) ON N 1s 2s 2p 2p
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Only d is t...View the full answer

Answered By

John Kimutai
I seek to use my competencies gained through on the job experience and skills learned in training to carry out tasks to the satisfaction of users. I have a keen interest in always delivering excellent work
4.70+
11+ Reviews
24+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
1. Which set of values is not correct for an electronoccupying a 4d orbital? A. n = 4, l = 2, ml = 0 B. n = 4, l = 2, ml = 1/2 C. n= 3, l = 4, ml = 1 D. n = 3, l = 1, ml = 1 2. Which set of quantum...
-
To what neutral atom do the following valence-shell configurations correspond? Indicate whether the configuration corresponds to the ground state or an excited state. (a) (b) () (d) 3.s 3s 3s N N 3.s...
-
Determine whether each of the following statements is true or false. If false, correct the statement to make it true: (a) The nucleus has most of the mass and comprises most of the volume of an atom....
-
Prepare an adjusted trial balance on May 31. Wildhorse's Hotel opened for business on May 1, 2022. Its trial balance before adjustment on May 31 is as follows. Account Number 101 126 130 140 141...
-
The cable AO exerts a force on the top of the pole of F = {?120i ? 90j ? 80k} lb. If the cable has a length of 34 ft, determine the height z of the pole and the location (x, y) of its base.
-
Kerry and Danielle wanted to investigate if tapping on a can of soda would reduce the amount of soda expelled after the can has been shaken. For their experiment, they vigorously shook 40 cans of...
-
Evaluate the risks and challenges of emerging markets. LO.1
-
Jan Dan Inc. (JDI) is a specialty frozen food processor located in the southeastern United States. Since its founding in 1992, JDI has enjoyed a loyal local clientele that is willing to pay premium...
-
Question 41 Not yet answered Marked out of 1.00 Flag question Mr. Ahmed is an auditor of Al Anwar Ceramics. Mr. Ahmed wants to collect more information about his new client's business before the...
-
Sodium vapor lamps, used for public lighting, emit yellow light of wavelength 589 nm. How much energy is emitted by (a) An excited sodium atom when it generates a photon; (b) 5.00 mg of sodium atoms...
-
Wavefunctions are normalized to 1. This term means that the total probability of finding an electron in the system is 1. Verify this statement for a particle-in-the-box wavefunction.
-
How are both beginning and ending goods in process inventories reported on a manufacturing statement?
-
Use the Comparison Theorem to determine whether the integral is convergent or divergent. L da
-
Problem 3 (2 scenarios) Scenario 1: Rocky Inc hired a new intern from CSU to help with year-end inventory. The intern computed the inventory counts at the end of 2020 and 2021. However, the intern's...
-
A CM reactor receives influent containing 10.0 mg/L of tracer for 2 h. Then tracer addition is terminated but the flow remains steady. The volume of the reactor is 10 L and the flow rate is 2 L / h....
-
Solve the given system of equations graphically by using a graphing calculator. y=5x x+y2=81 Find the solution with the smaller x-value. x= y= (Type an integer or a decimal rounded to one decimal...
-
I-The market for Sony's Playstation5 game console has changed from 2021 to 2023. With restrictions from the Covid-19 pandemic ending people are finding other entertainment options available such as...
-
Solve. a. 12 = x - 8/x+3 b. 21 = 3x + 8/x + 5 c. 3 = 2x + 5/4x -7 d. - 4 = -6x + 5/2x + 3
-
Explain the Hawthorne effect.
-
Amino acids can act as ligands toward transition metal ions. The simplest amino acid is glycine (NH2CH2CO2H). Draw a structure of the glycinate anion (NH2CH2CO2-), acting as a bidentate ligand. Draw...
-
The carbonate ion (CO32-) can act as either a monodentate or a bidentate ligand. Draw a picture of CO32- coordinating to a metal ion as a bidentate and as a monodentate ligand. The carbonate ion can...
-
Which of the following ligands are capable of linkage isomerism? Explain your answrer. SCN, N3, NO2, NH2CH CH2NH2, OCN-,I
-
The following schedule reconciles Cele Co.'s pretax GAAP income Pretax GAAP income Nondeductible expense for fines Tax deductible depreciation in excess of GAAP depreciation expens Taxable rental...
-
How to solve general ledger cash balance chapter 9 assignment 5
-
On 31 July 2018, Sipho bought 1 000 ordinary shares in ABC Ltd at a cost of R2 750. On 31 December 2018 the company made a 1 for 10 bonus issue. On 31 March 2019, Sipho sold 300 shares for R800. What...

Study smarter with the SolutionInn App


