Question: Diborane (B 2 H 6 ) is a highly reactive boron hydride that was once considered as a possible rocket fuel for the U.S. space
Diborane (B2H6) is a highly reactive boron hydride that was once considered as a possible rocket fuel for the U.S. space program. Calculate ΔH for the synthesis of diborane from its elements, according to the equation
![]()
using the following data:
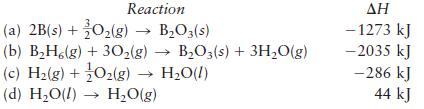
2B(s) + 3H(g) - BH6(g)
Step by Step Solution
3.39 Rating (149 Votes )
There are 3 Steps involved in it
To obtain AH for the required reaction we must somehow com bine equations a b c and d to produce tha... View full answer

Get step-by-step solutions from verified subject matter experts


