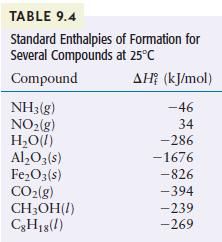
Using the standard enthalpies of formation listed in Table 9.4, calculate the standard enthalpy change for the
Question:
Using the standard enthalpies of formation listed in Table 9.4, calculate the standard enthalpy change for the overall reaction that occurs when ammonia is burned in air to form nitrogen dioxide and water. This is the first step in the manufacture of nitric acid.
![]()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





