Methanol (CH 3 OH) is sometimes used as a fuel in high-performance engines. Using the data in
Question:
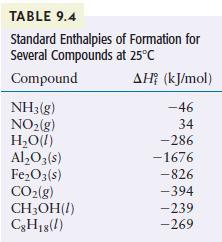
Methanol (CH3OH) is sometimes used as a fuel in high-performance engines. Using the data in Table 9.4, compare the standard enthalpy of combustion per gram of methanol with that of gasoline. Gasoline is actually a mixture of compounds, but assume for this problem that gasoline is pure liquid octane (C8H18).
Transcribed Image Text:
TABLE 9.4 Standard Enthalpies Several Compounds at 25C Compound NH3(g) NO(g) HO(1) AlO3(s) FeO3(s) CO(g) CHOH(1) C8H18(1) of Formation for AH (kJ/mol) -46 34 -286 -1676 -826 -394 -239 -269
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The combustion reaction for methanol is 2CHOH1 30g 2COg 4HOl Using the standard enthalpies of format...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Methanol (CH3OH) has also been proposed as an alter-native fuel. Calculate the standard enthalpy of combustion per gram of liquid methanol, and compare this answer to that for ethanol in Exercise 80.
-
Methanol (CH3OH) is used as a fuel in race cars. (a) Write a balanced equation for the combustion of liquid methanol in air. (b) Calculate the standard enthalpy change for the reaction, assuming...
-
In January 1995, the Office of University Evaluation at Arizona State University surveyed faculty and staff members to find out their reaction to the closure of the university during Winter Break,...
-
Glass bottles can be either recycled (crushed and re-melted) or reused. The market will tend to choose the cheapest path. What factors will tend to affect the relative cost of these options? Is the...
-
Let the value of a in the logistic equation, Equation 4.46, be equal to 0.9, Make a map like that in Figure 4-21 when x1 = 0.4. Make the plot for three other values of x1 for which 0 < x1 < 1.
-
Obtain the Laplace transform of the function plotted in Figure. f(T) 2D
-
7 Outline the stages through which organisations go in using the internet, giving an original example of each.
-
Consider the following transactional data for the first month of operations of Shine King Cleaning, Inc. Nov 1: Evan Hudson deposited $35,000 in the business account. Also on this date, Evan...
-
please show how to get the pv either in sceduel or finacial calculator On Jan 1 2019 LCM2 issued a 7 year $700,000 Bond paying 8% interest semi-annually. The market rate for bonds of similar risk and...
-
Assuming that the combustion of hydrogen gas provides three times as much energy per gram as gasoline, calculate the volume of liquid H 2 (density = 0.0710 g/mL) required to furnish the energy...
-
Using the standard enthalpies of formation listed in Table 9.4, calculate the standard enthalpy change for the overall reaction that occurs when ammonia is burned in air to form nitrogen dioxide and...
-
Identify the requested amount in each of the following situations: a. MaryAnn Company's August 31 bank reconciliation shows deposits in transit of \(\$ 2,250\). The general ledger Cash in Bank...
-
Compare the alternatives that Bergerac is considering for its decision. Include: Comparison of make versus buy option in the type of operation that Bergerac is looking to integrate. You do not need...
-
Let A, B, C and D be non-zero digits, such that CD is a two-digit positive integer. BCD is a three-digit positive integer generated by the digits B, C and D. ABCD is a four-digit positive integer...
-
1.) An aluminum tube is clamped with rigid plates using four bolts as shown. The nut on each bolt is tightened one turn from 'snug'. The thickness of the plate may be considered insignificant in this...
-
4.21 Case Study Competency IV.1RM Determine diagnosis and procedure codes and groupings according to official guidelines. Competency IV.1 Validate assignment of diagnostic and procedural codes and...
-
W.E.B Dubois taught the book called "The State" to his students at Atlanta University. Who wrote this book
-
Consider a simple Brayton cycle using air as the working fluid; has a pressure ratio of 12; has a maximum cycle temperature of 600°C; and operates the compressor inlet at 100 kPa and 15°C....
-
You deposit $10,000 in a savings account that earns 7.5% simple interest per year. What is the minimum number of years you must wait to double your balance? Suppose instead that you deposit the...
-
Write chemical equations for (a) The burning of lithium in oxygen; (b) The reaction of sodium metal with water; (c) The reaction of fluorine gas with water; (d) The oxidation of water at the anode of...
-
Identify the oxidation number of the halogen in (a) Hypoiodous acid; (b) ClO 2 ; (c) Dichlorine heptoxide; (d) NaIO 3 .
-
What are the sources for the production of helium and argon?
-
On NSE (Indian stock exchange), shares of ICICI Bank trade for 935 rupees. If the spot exchange rate is USD 0.012, what is the no-arbitrage USD price of ICICI Bank ADR? Assume that transactions costs...
-
Income Statement Balance Sheet Balance Sheet Additional Financial Information 1. Market price of Ranfield's common stock: $90.44 at December 31, 2024, and $58.35 at December 31, 2023. 2. Common...
-
There is a credit rating agency for businesses that gives out various amounts of information based on the subscription level. This company is called a. Business Credit Scoring b. Fair Issue c. Dun...

Study smarter with the SolutionInn App


