During the analysis of an unknown acid HA, a 0.010 m solution of the sodium salt of
Question:
During the analysis of an unknown acid HA, a 0.010 m solution of the sodium salt of the acid was found to have a pH of 10.35. Use Table 6C.1 to identify the acid.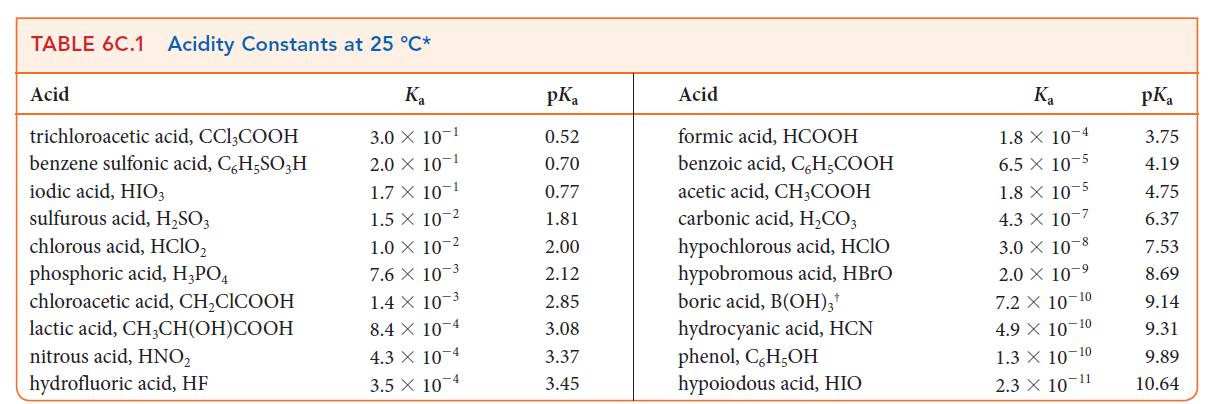
Transcribed Image Text:
TABLE 6C.1 Acidity Constants at 25 °C* K₂ 3.0 X 10-1 2.0 X 10-1 1.7 X 10-1 1.5 X 10-2 1.0 × 10-2 7.6 X 10-3 1.4 x 10-3 8.4 X 10-4 4.3 X 10-4 3.5 x 10-4 Acid trichloroacetic acid, CC13COOH benzene sulfonic acid, C,H,SO3H iodic acid, HIO3 sulfurous acid, H₂SO3 chlorous acid, HClO₂ phosphoric acid, H3PO4 chloroacetic acid, CH₂CICOOH lactic acid, CH₂CH(OH)COOH nitrous acid, HNO₂ hydrofluoric acid, HF pK₂ 0.52 0.70 0.77 1.81 2.00 2.12 2.85 3.08 3.37 3.45 Acid formic acid, HCOOH benzoic acid, C,H,COOH acetic acid, CH₂COOH carbonic acid, H₂CO3 hypochlorous acid, HCIO hypobromous acid, HBrO boric acid, B(OH)3¹ hydrocyanic acid, HCN phenol, C,H,OH hypoio dous acid, HIO K₂ 1.8 X 10 4 6.5 x 10-5 1.8 x 10-5 4.3 X 10-7 3.0 X 10-8 2.0 × 10-9 7.2 X 10-10 4.9 X 10-10 1.3 X 10-10 2.3 × 10 11 pKa 3.75 4.19 4.75 6.37 7.53 8.69 9.14 9.31 9.89 10.64
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
The fo...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
During the analysis of an unknown weak base B, a 0.10 m solution of the nitrate salt of the base was found to have a pH of 3.13. Use Table 6C.2 to identify the base. TABLE 6C.2 Basicity Constants at...
-
The partnership agreement of Axel, Berg & Cobb provides for the year-end allocation of net income in the following order: First, Axel is to receive 10% of net income up to $100,000 and 20% over...
-
Can we use servomotor for position control? Support the answer with necessary details
-
A manufacturer claims that the average tensile strength of thread A exceeds the average tensile strength of thread B by at least 12 kilograms. To test his claim, 50 pieces of each type of thread are...
-
If a loop of chain is spun at high speed, it will roll like a hoop without collapsing. Consider a chain of linear mass density m that is rolling without slipping at a high speed v 0 . (a) Show that...
-
Calculate the magnitude of the diffusion-controlled rate constant at 298 K for the recombination of two atoms in benzene, for which 17 = 0.601 cP. Assuming the concentration of the reacting species...
-
(a) Prove that f+(x) ~ 0, f-(x) ~ 0, f(x) = f+(x) - f-(x), and If(x)1 = f+(x) + f-(x) hold for all x E Dom (f). (Compare with Exercise 1, p. 11.) (b) Prove that if L = lim f(x) x-->a exists, then f+...
-
Discuss specific steps you would take as a manager to ensure that a business process reengineering effort is successful.
-
Which of the following is an Amortization: A. Is the systematic allocation of the cost of an intangible asset to expense over its estimated useful life B. Is the process of allocating to expense the...
-
Mike Curtains, a Registered Tax Agent, attends to the tax affairs of Frodo West. In preparing Frodo's 2021/22 income tax return, the following occurred: Frodo attended a meeting with Mike in August...
-
Calculate the molar concentration of H 3 O + in solutions with the following molar concentrations of OH : (a)0.0021 mol L 1 ; (b) 3.4 * 10 3 mol L 1 ; (c)7.60 mmol L 1
-
Decide which acid in each of the following pairs is the stronger and explain why: (a) H 3 AsO 4 or H 3 PO 4 ; (b) HBrO 3 or HBrO; (c) H 3 PO 4 or H 3 PO 3 ; (d) H 2 Te or H 2 Se; (e) H 2 S or HCl;...
-
Let S(n) = 1 + 2 + + n be the sum of the first n natural numbers and let C(n) = 1 3 + 2 3 + + n 3 be the sum of the first n cubes. Prove the following equalities by induction on n, to arrive at...
-
# III: Worksheet 3 1. A 20 kg mass is allowed to accelerate down a frictionless 15 ramp. 20 kg 15 a. Draw a force diagram for the block. b. Determine the value of the x-component of the force of...
-
3.Baker Corporation has provided the following production and average cost data for two levels of monthly production volume. The company produces a single product Production Volume: 1,000 units:...
-
Suppose that you own the only company in the market to produce a certain product, and therefore you can determine the market price P dollars for each unit. Due to government regulations, the price of...
-
describes how the blast pressure front can bounce off solid, immovable obstacles and be redirected in another direction in a linear angle to the angle of the obstacle hat was struck
-
As the accounting clerk, you are tasked by the CFO to determine the cost of goods sold of Del Mundo Company for the year ended December 31, 2020. During Operating cost data annd inventory account...
-
How is the syndrome for the Hamming code interpreted?
-
Problem 2. (0.6 points, 0.2 points for each question) (a) A company turns its inventory 2 times a month. Its months-of-supply = Its days-of-supply = Please show your analysis below: _months. days. (1...
-
Many processes such as the fabrication of integrated circuits are carried out in a vacuum chamber to avoid reaction of the material with oxygen in the atmosphere. It is difficult to routinely lower...
-
A 3.50 mole sample of N 2 in a state defined by T i = 250.K and V i = 3.25 L undergoes an isothermal reversible expansion until V f = 35.5 L. Calculate w assuming a. That the gas is described by the...
-
A major league pitcher throws a baseball with a speed of 162 kilometers per hour. If the baseball weighs 235 grams and its heat capacity is 1.7 J g 1 K 1 , calculate the temperature rise of the ball...
-
Just work out the assignment on your own sheet, you dont need the excel worksheet. Classic Coffee Company Best friends, Nathan and Cody, decided to start their own business which would bring great...
-
Financial information related to the proprietorship of Ebony Interiors for February and March 2019 is as follows: February 29, 2019 March 31, 2019 Accounts payable $310,000 $400,000 Accounts...
-
(b) The directors of Maureen Company are considering two mutually exclusive investment projects. Both projects concern the purchase of a new plant. The following data are available for each project...

Study smarter with the SolutionInn App


