During the analysis of an unknown weak base B, a 0.10 m solution of the nitrate salt
Question:
During the analysis of an unknown weak base B, a 0.10 m solution of the nitrate salt of the base was found to have a pH of 3.13. Use Table 6C.2 to identify the base.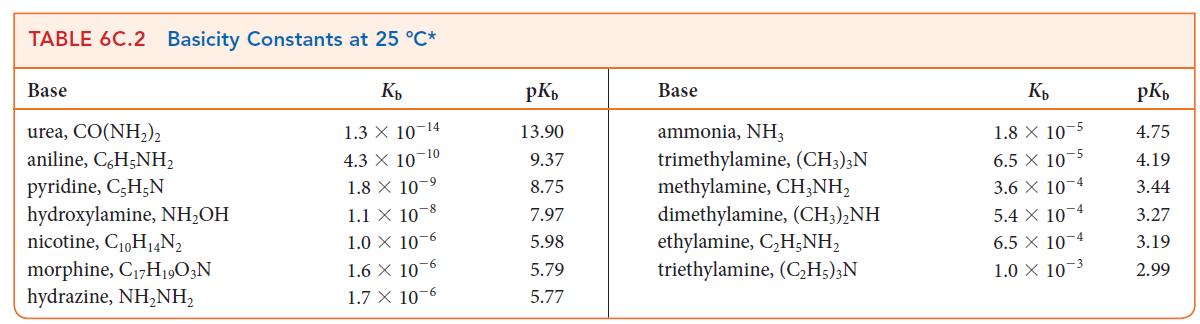
Transcribed Image Text:
TABLE 6C.2 Basicity Constants at 25 °C* Kb 1.3 X 10-14 4.3 X 10-10 1.8 X 10-9 1.1 X 10 8 1.0 x 10-6 1.6 x 10-6 1.7 X 10-6 Base urea, CO(NH,) aniline,C6H5NH₂ pyridine, C,H-N hydroxylamine, NH₂OH nicotine, C₁0H₁4N₂ morphine, C₁7H1903N hydrazine, NH₂NH₂ pKb 13.90 9.37 8.75 7.97 5.98 5.79 5.77 Base ammonia, NH3 trimethylamine, (CH3)3N methylamine, CH3NH₂ dimethylamine, (CH3)2NH ethylamine, C₂H₂NH₂ triethylamine, (C₂H5)3N Kb 1.8 X 10 5 6.5 X 105 3.6 X 10-4 5.4 x 10-4 6.5 x 10-4 1.0 X 10-3 pKb 4.75 4.19 3.44 3.27 3.19 2.99
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To identify the base B using the pH of its nitrate salt solution and Table 6C2 we need to understand the relationship between pH pOH and the pKb of th...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
During the analysis of an unknown acid HA, a 0.010 m solution of the sodium salt of the acid was found to have a pH of 10.35. Use Table 6C.1 to identify the acid. TABLE 6C.1 Acidity Constants at 25...
-
A student dissolves 0.0100 mol of an unknown weak base in 100.0 mL water and titrates the solution with 0.100 M HNO 3 . After 40.0 mL of 0.100 M HNO 3 was added, the pH of the resulting solution was...
-
Use Table 6H.2 to suggest suitable indicators for the titrations described in Exercises 6H.10 and 6H.12. Exercises 6H.10 Morphine, C 17 H 19 O 3 N, is a potent painkiller. Suppose you are studying...
-
What type of isomers are exhibited by [Fe(en) 3 ]Cl 2 (en = ethane-1,2-diamine)? no isomers are possible. cis and trans isomers fac and mer isomers optical isomers
-
In this problem you will derive an expression for the potential energy of a segment of a string carrying a traveling wave (Figure). The potential energy of a segment equals the work done by the...
-
Sucrose is readily hydrolyzed to glucose and fructose in acidic solution. The hydrolysis is often monitored by measuring the angle of rotation of plane polarized light passing through the solution....
-
(i) Prove that f(x) = { x+l x-I x~o x
-
The ledger of Danieal Rental Agency on March 31 of the current year includes the selected accounts, shown on the next page, before adjusting entries have been prepared. An analysis of the accounts...
-
This year Elizabeth agreed to a three-year service contract with an engineering consulting firm to improve efficiency in her factory. The contract requires Elizabeth to pay the consulting firm $1,450...
-
Fawcett Institute provides one-on-one training to individuals who pay tuition directly to the business and also offers extension training to groups in off-site locations. Fawcett prepares adjusting...
-
The value of K w for water at body temperature (37C) is 2.1 * 10 14 . (a) What is the molar concentration of H 3 O + ions at 37C? (b) What is the molar concentration of OH in neutral water at 37 C?
-
Suggest an explanation for the different strengths of (a) Acetic acid and trichloroacetic acid; (b) Acetic acid and formic acid.
-
Cramer's rule can be applied to systems of three linear equations in three variables. For the system of equations the solution can be written as follows. If D 0 a unique solution exists and is given...
-
FA II: Assignment 1 - COGS & Bank Reconciliation 1. The following data pertains to Home Office Company for the year ended December 31, 2020: Sales (25% were cash sales) during the year Cost of goods...
-
Bramble Stores accepts both its own and national credit cards. During the year, the following selected summary transactions occurred. Jan. 15 20 Feb. 10 15 Made Bramble credit card sales totaling...
-
11. Korina Company manufactures products S and T from a joint process. The sales value at split-off was P50000 for 6,000 units of Product S and P25,000 for 2,000 units of Product T. Assuming that the...
-
Karak Company produces Product (A) for only domestic distribution since year 2017. In 2019, a similar product to Karak Company has come onto the market by another competitor. Karak Company is keen to...
-
1. Purchase equipment in exchange for cash of $20,400. 2. Provide services to customers and receive cash of $4,900. 3. Pay the current month's rent of $1,000. 4. Purchase office supplies on account...
-
What are the differences among EPROM, EEPROM, and flash memory?
-
Arlington Merchants reported the following on its income statement for the fiscal years ending December 31, 2016 and 2015. 2016 2015 Sales $4,857,500 $4,752,900 Cost of goods sold 3,258,950 3,207,000...
-
A glass bulb of volume 0.198 L contains 0.457 g of gas at 759.0 Torr and 134.0C. What is the molar mass of the gas?
-
Use LHpitals rule lim [f(x)/g(x)] x 0 = lim [df(x)dx/dg(x)/dx] x 0 to show that the expression derived for Pf in part b of Example problem 1.1 hane the correct limit as y 0.
-
For each compound below, identify all lone pairs and indicate whether each lone pair is localized or delocalized. Then, use that information to determine the hybridization state and geometry for each...
-
Selected comparative financial statement data for DAS inc. Balance Sheet (En milliers de dollars) 2017 2018 Assets Assets CT - Cash 41.63 47.5 - Accounts Receivable 64.2 72.6 - inventories 969.7...
-
please help!! One chance at turning in!!! 16 rows! I'd highly appreicate it I am unsure what information you need... I provided all Current Attempt in Progress Mike Greenberg opened Grouper Window...
-
Blue Ridge Marketing Inc. manufactures two products, A and B . Presently, the company uses a single plantwide factory overhead rate for allocating overhead to products. However, management is...

Study smarter with the SolutionInn App


