Each of the following valence-shell configurations is possible for a neutral atom of a certain element. What
Question:
Each of the following valence-shell configurations is possible for a neutral atom of a certain element. What is the element and which configuration represents the ground state?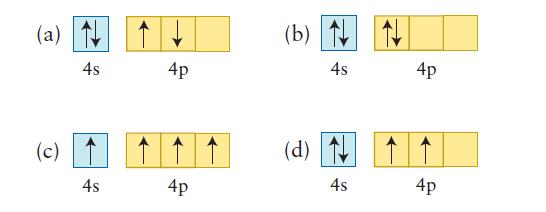
Transcribed Image Text:
(a) N 4s (c) 4s 4p 4p (b) N 4s (d) 4s 4p 4p
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The image youve provided shows four different electron configurations for the valence shells outermost energy levels of an atom Each configuration has ...View the full answer

Answered By

Jacob Festus
I am a professional Statistician and Project Research writer. I am looking forward to getting mostly statistical work including data management that is analysis, data entry using all the statistical software’s such as R Gui, R Studio, SPSS, STATA, and excel. I also have excellent knowledge of research and essay writing. I have previously worked in other Freelancing sites such as Uvocorp, Essay shark, Bluecorp and finally, decided to join the solution inn team to continue with my explicit work of helping dear clients and students achieve their Academic dreams. I deliver, quality and exceptional projects on time and capable of working under high pressure.
4.90+
1263+ Reviews
2858+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
1 . What are characteristics of a bond and what is the purpose of it ? 2 . What determines the Bond Price? 3 . What is the relationship between Price of a Bond and its return? What if the Price of a...
-
1) Match each of the following electron configurations to the correct atom. 1s 2 2s 2 2p 2 1s 2 2s 2 2p 6 3s 1 1s 2 2s 2 2p 6 3s 2 3p 5 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 3 1s 2 2s 2 2p 6 3s 2 3p 6 4s...
-
The beam shown below is supported by a pin at A and a roller at B. The weight of the beam is 12 kN and a 15 kN force is applied 3 m to the right of A as shown. Determine the reaction forces at A and...
-
The cylindrical plate is subjected to the three cable forces which are concurrent at point D. Express each force which the cables exert on the plate as a Cartesian vector, and determine the magnitude...
-
Use your calculators RandInt function to generate 200 digits from 0 to 9 and store them in a list. a. State appropriate hypotheses for a chi-square test for goodness of fit to determine whether your...
-
Which of the following is a characteristic of a highly involved consumer? a. Greater interest in the product b. Spending more time researching the product c. Engaging in extensive problem solving d....
-
Langer Company has three products (A, B, and C) that use common facilities. The relevant data concerning these three products follow. Required If fixed costs allocated to product line C are not...
-
You are a CPA at a local accounting firm. Review the scenario below: Carrie and Joe are married with two children, ages 2 and 7. They both work. Carrie owns a manufacturing business, with a net...
-
The wavefunction for a particle in a one- dimensional box is given in Eq. 2. Does the probability of finding the particle in the left-hand one-third of the box depend on n ? If so, find the...
-
When an electron beam strikes a block of copper, x-rays with a frequency of 1.2 * 10 17 Hz are emitted. How much energy is emitted at this wavelength by (a) An excited copper atom when it generates...
-
Denoting by st the static deflection of a beam under a given load, show that the frequency of vibration of the load is Neglect the mass of the beam, and assume that the load remains in contact with...
-
Q13. The probability that Ryan will roll a three using a standard die is 1/6. Let Y = number of times that Ryan has to roll a die in order to roll the first three. What is the expected value for Y?...
-
1. The following are data for two IT projects for a new database system. Prepare a spreadsheet for two projects, using the following data. Amounts are in thousands of dollars. Calculate the NPV for...
-
The Matsui Lubricants plant uses the weighted-average method to account for its work-in-process inventories. The accounting records show the following information for a particular day: Beginning WIP...
-
James Cook, a production department worker, is paid on hourly basis at a rate of $15 per hour. James works 40 hours per week. Any time James works over 40 hours, it is considered as overtime and he...
-
You just started working as a Health Service Manager within one of the following healthcare industries. First, choose an industry below to discuss the questions that follow: Ambulatory Surgery center...
-
Consider the equation y = (x - 1) (x + 4) / (x - 2) (x + 3). a. Describe the features of the graph of this function. b. Describe the end behavior of the graph. c. Sketch the graph.
-
For the following exercises, rewrite the sum as a product of two functions or the product as a sum of two functions. Give your answer in terms of sines and cosines. Then evaluate the final answer...
-
You travel to a distant, cold planet where the ammonia flows like water. In fact, the inhabitants of this planet use ammonia (an abundant liquid on their planet) much as earthlings use water. Ammonia...
-
Provide a reasonable estimate for the number of atoms in a 150-lb adult human. Use the information given in Table 18.2. Table 18.2 Trace Elements Major Elemens Mass Percent in alphabetical order)...
-
Lead forms compounds in the 12 and 14 oxidation states. All lead(II) halides are known (and are known to be ionic). Only PbF4 and PbCl4 are known among the possible lead(IV) halides. Presumably lead...
-
C0 = 10.648148 b) ( 4 Marks ) As of now, Given the above conditions on the option, what is the intrinsic value of the call option? What is the time value of the call option?
-
interest revenue 19,500 retained earning,end 5,000 selling expenses 145,00 prepaid insurance 20,000 loss and disposal of a business (discountied),net 28,000 income from operation 140,000 unearned...
-
cost that do not extend the acid capacity or it's useful life, but merely maintained the assd, or restore it to working order are recorded as losses True or False

Study smarter with the SolutionInn App


