Each of the pictures below shows a molecular view of a system undergoing a change. In each
Question:
Each of the pictures below shows a molecular view of a system undergoing a change. In each case, indicate whether heat is absorbed or given off by the system and whether expansion work is done on or by the system. Predict the signs of q and w for the process.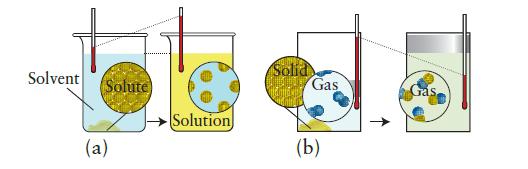
Transcribed Image Text:
Solvent Solute (a) Solution Solid Gas (b)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a A solid is dissolving in solution and as indicated there is no volu...View the full answer

Answered By

Douglas Makokha
Unlock Academic Success with Dedicated Tutoring and Expert Writing Support!
Are you ready to excel in your academics? Look no further! As a passionate tutor, I believe that dedication and hard work are the keys to achieving outstanding results. When it comes to academics, I strive to provide nothing but the best for every student I encounter.
With a relentless thirst for knowledge, I have extensively researched numerous subjects and topics, equipping myself with a treasure trove of answers to tackle any question that comes my way. With four years of invaluable experience, I have mastered the art of unraveling even the most intricate problems. Collaborating with esteemed writers has granted me exclusive access to the trade secrets utilized by the industry's top professionals.
Allow me the pleasure of assisting you with your writing assignments. I thrive on challenges and will guide you through any obstacles you may face. Together, we will unlock your academic potential and pave the way for your success.
4.90+
62+ Reviews
349+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Summarize each data source and include them for substance abuse and alcohol in the military. Analyze each data source for substance abuse and alcohol in the military for trustworthiness and accuracy....
-
Each of the pictures below shows a molecular view of a system undergoing a change at constant temperature. In each case, indicate whether heat is absorbed or released by the system and whether...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Jinny Buffett recently retired as a flight attendant and is interested in opening a fitness center and health spa exclusively for women in Grand Cayman, where she resides. After careful study, she is...
-
For the year ended December 31, 2010, the job cost sheets of Moxie Company contained the following data. Other data:1. Raw materials inventory totaled $20,000 on January 1. During the year, $100,000...
-
Use the Table of Integrals on Reference Pages 610 to evaluate the integral. cot x =dx V1 + 2 sin x
-
4. Dar invests $90,000 cash in the partnership for a 30% interest in the capital and profits, and partnership assets are not revalued.
-
Water at 20C and a flow rate of 0.1 kg/s enters a heated, thin-walled tube with a diameter of 15 mm and length of 2 m. The wall heat flux provided by the heating elements depends on the wall...
-
please help Required Information {The following information applies to the questions displayed below) Most Company has an opportunity to invest in one of two new projects. Project Y requires a...
-
If you start with 276 g of liquid water at 25.C, how much heat must be supplied to convert all the liquid into vapor at 100.C?
-
Calculate the change in entropy when the pressure of 5.75 g of helium gas is decreased from 320.0 kPa to 40.0 kPa while the temperature decreases from 423 K to 273 K. Assume ideal behavior.
-
For the data in Exercise 8.13, the ticket requests received are given in Table 8.6. Determine whether or not to accept each one, and update the bid prices after each transaction. Only accept a...
-
The problem I have identified is that healthcare leaders could benefit from addressing the issue of stress and burnout, which impact revenue (Scott, 2022). I have found a peer-reviewed article...
-
Facebook, Inc is the company Complete a 3-5 year forecast for your target company assuming a 10% average growth rate for the duration of the forecast period Assuming a long-term growth rate of 5%...
-
BSC-It is important for healthcare leaders to link their departmental balanced scorecard (BSC) to a corporate BSC because it facilitates alignment with the overall strategic objectives of the...
-
Hebert Company adds material at the beginning of production. The following production information is available for March: Beginning Work in Process Inventory (40% complete as to conversion) Started...
-
What modifications would you suggest the leaders of the steel organization when dealing with the use of more efficient technology, carbon emissions, and negative economic impacts in order tomake in...
-
In the clover butterfly, males are always yellow, but females can be yellow or white. In females, white is a dominant allele. Two yellow butterflies were crossed to yield an F1 generation consisting...
-
Refrigerant R-12 at 30C, 0.75 MPa enters a steady flow device and exits at 30C, 100 kPa. Assume the process is isothermal and reversible. Find the change in availability of the refrigerant.
-
Which of the following statements is(are) true? Correct the false statement(s). a. When a reactant is added to a system at equilibrium at a given temperature, the reaction will shift right to...
-
Suppose the reaction system UO2(s) + 4HF(g) UF4(g) + 2H2O(g) has already reached equilibrium. Predict the effect that each of the following changes will have on the equilibrium position. Tell...
-
Consider the reaction: Fe3+(aq) + SCN2(aq) FeSCN2+(aq) How will the equilibrium position shift if a. Water is added, doubling the volume? b. AgNO3(aq) is added? (AgSCN is insoluble.) c. NaOH(aq) is...
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


