Each of the pictures below shows a molecular view of a system undergoing a change at constant
Question:
Each of the pictures below shows a molecular view of a system undergoing a change at constant temperature. In each case, indicate whether heat is absorbed or released by the system and whether expansion work is done on or by the system. Predict the signs of q and w for the process.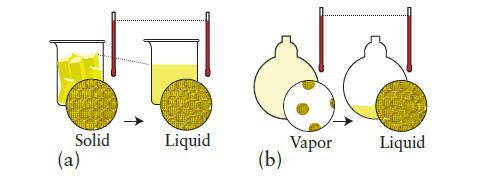
Transcribed Image Text:
Solid (a) T Liquid (b) Vapor Liquid
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (4 reviews)
a Absorbed by the sys...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Each of the pictures below shows a molecular view of a system undergoing a change. In each case, indicate whether heat is absorbed or given off by the system and whether expansion work is done on or...
-
(Multiple choice) (1) The temperature change of two blocks of masses MA and MB is the same when they absorb equal amounts of heat. It follows that the specific heats are related by (a) cA =...
-
Consider the expansion of a gas at constant pressure in a water-cooled pistoncylinder system. The constant pressure is achieved by controlled input of energy as heat Q to the gas. Treating the gas as...
-
Quick Fix-it Corporation was organized in January 2011 to operate several car repair businesses in a large metropolitan area. The charter issued by the state authorized the following capital stock:...
-
Easy Decorating uses a job order costing system to collect the costs of its interior decorating business. Each clients consultation is treated as a separate job. Overhead is applied to each job based...
-
Evaluate the integral. S sin(1 + x) dx
-
PR 16-2 Under what circumstances can a partnership expel a partner?
-
Howe and Duleys company is organized as a partnership. At the prior year- end, partnership equity totaled $150,000 ($100,000 from Howe and $50,000 from Duley). For the current year, partnership net...
-
1. X Co reports: Cash Inflows from operating activities 125,000 Cash outflows from investing activities 60,000 Cash inflows from financing activities 70,000 ending cash balance 200,000 What is x co,...
-
During the test of an internal combustion engine, 3.00 L of nitrogen gas at 18.5 C was compressed suddenly (and irreversibly) to 0.500 L by driving in a piston. In the process, the temperature of the...
-
How much heat is needed to convert 80.0 g of ice at 0.0 C into liquid water at 20.0C?
-
Write up the asset and liability and capital accounts to record the following transactions in the records of F Murray. 20X7 July "1 11 1 Started business with 15,000 in the bank. 2 Bought office...
-
The waiting times between a subway departure schedule and the arrival of a passenger are uniformly distributed between 0 and 9 minutes. Find the probability that a randomly selected passenger has a...
-
Greenview Dairies produces a line of organic yogurts for sale at supermarkets and specialty markets in the Southeast. Economic conditions and changing tastes have resulted in slowing demand growth....
-
Rudy Gandolfi owns and operates Rudy's Furniture Emporium Inc. The balance sheet totals for assets, liabilities, and stockholders' equity at August 1, 2019, are as indicated. Described here are...
-
If you were team leader how would you break up this assignment for 4 people to complete? Group Case Analysis Parts 4, 5, and 6 IV. STRATEGY IMPLEMENTATION. (How are you going to do what you want to...
-
A genetic experiment with peas resulted in one sample of offspring that consisted of 440 green peas and 166 yellow peas. Construct a 90% confidence interval to estimate of the percentage of yellow...
-
Certain species of summer squash exist in long, spherical, or disk shapes. When a true-breeding long-shaped strain was crossed to a true-breeding disk-shaped strain, all of the F1 offspring were...
-
Complete the equations for the following equilibria and calculate Keq where the Keq expression includes [HO]. Be sure to enter Keq in proper scientific notation. (a) ammonia (acting as a base) reacts...
-
A 4.72- g sample of methanol (CH3OH) was placed in an otherwise empty 1.00- L flask and heated to 250oC to vaporize the methanol. Over time the methanol vapor decomposed by the following reaction:...
-
The compound SbCl5(g) decomposes at high temperatures to gaseous antimony trichloride and chlorine gas. When 89.7 g of SbCl5(g) is placed in a 15.0-L container at 1808C, the SbCl5(g) is 29.2%...
-
At 207oC, Kp = 0.267 for the reaction PCl5(g) PCl3(g) + Cl2(g) a. If 0.100 mole of PCl5(g) is placed in an otherwise empty 12.0-L vessel at 207oC, calculate the partial pressures of PCl5(g),...
-
you are analyzing the cost of debt for a firm. Do you know that the firms 14 year maturity, 7.8 Percent coupon bonds are selling at a price of $834. The Barnes pay interest semi annually. If these...
-
***Please answer the following using excel and showcasing the formulas/calculations used*** thank you so much Financial information on AAA Ltd. is shown below. AAA Ltd. Income Statement For the Year...
-
2. In an account Anh Paglinawan currently has $216,670.00. At a rate of 8.00% how long will it take for them to have $298,390.00 assuming semi-annually compounding? (Hint: compute the exact years, do...

Study smarter with the SolutionInn App


