Estimate the density of each of the following solids from the ionic radii given in Fig. 1F.6:
Question:
Estimate the density of each of the following solids from the ionic radii given in Fig. 1F.6:
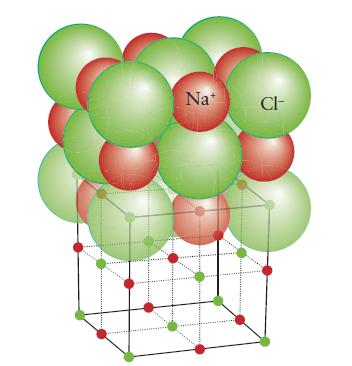
(a) Calcium oxide (rock-salt structure, Fig. 3H.28);
(b) Cesium bromide (cesium- chloride structure, Fig. 3H.30).
FIGURE 3H.30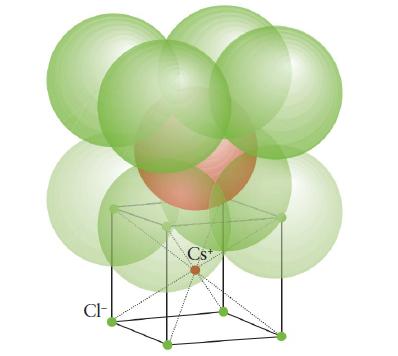
FIGURE 3H.28
Transcribed Image Text:
CI- Cs+
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a d 3...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The table below lists the ionic radii for the cations and anions in three different ionic compounds. Each compound has either the NaCl, CsCl, or ZnS type cubic structure. Predict the type of...
-
From Equation 8.4 and the ionic radii given in Figure 7.7, calculate the potential energy of the following pairs of ions. Assume that the ions are separated by a distance equal to the sum of their...
-
From the ionic radii given in Figure 7.7, calculate the potential energy of a Ca2+ and O2- ion pair that is just touching (the magnitude of the electronic charge is given on the back inside cover)....
-
2. Evaluate the following definite integrals (a) (2x - 6x) dx /6 (b) 16 cos 3t + 2 sin 3t dt x+1 2 da
-
Replace the two forces acting on the grinder by a resultant force and couple moment at point O. Express the results in Cartesian vectorform. F, = [101 - 15j - 40k} N 250 mm F; = |-151 20j 30OK} N...
-
How much storage space does your music use? Here is a dotplot of the file sizes (to the nearest tenth of a megabyte) for 18 randomly selected files on Nathaniels mp3 player: a. The distribution of...
-
2. There is a single debt issue. Compute the yield on this debt assuming that it matures in 1 year and has a maturity value of \($127.42\), 2 years with a maturity value of \($135.30\), 5 years with...
-
The following information, in T-account format, is provided for Mars Company for the year 2012: Additional information: a. Sales revenue for the period was $164,000. Accounts receivable (net)...
-
Nash Corporation wishes to exchange a machine used in its operations. Nash has received the following offers from other companies in the industry. 1. Crane Company offered to exchange a similar...
-
Metals with bcc structures, such as tungsten, are not close packed. Therefore, their densities would be greater if they were to change to a ccp structure (under pressure, for instance). What would...
-
Without doing a calculation, order the following gases according to increasing mass density: N 2 H 4 ; N 2 ; NH 3 . The temperature and pressure are the same for all three samples.
-
Calculate the pH of a solution formed by mixing 100.0 mL of 0.100 M NaF and 100.0 mL of 0.025 M HCl.
-
Photon Technologies, Inc., a manufacturer of batteries for mobile phones, signed a contract with a large electronics manufacturer to produce three models of lithium-ion battery packs for a new line...
-
Mastery Problem: Capital Investment Analysis HomeGrown Company HomeGrown Company is a chain of grocery stores that are similar to indoor farmer's markets, providing fresh, local produce, meats, and...
-
McDonald's and CSR There more than 32,000 restaurants around the world (www.aboutmcdonalds.com/etc/medialib/csr/docs. that carry the McDonald's label and logo. As such, they...
-
Smartwatch Based on a survey by Consumer Technology Association, smartwatches are used in 18% of U.S. households. Find the probability that a randomly selected U.S. household has no smartwatches.
-
Suppose you wanted to purchase a commercial real estate property thats valued at $ 1 , 0 0 0 , 0 0 0 . You could secure financing from a traditional bank, which provides you with $ 7 5 0 , 0 0 0 ....
-
The path traced by a fixed point, P, on a moving wheel is called a cycloid and is shown below. A cycloid can be defined with parametric equations. Use the diagram to derive parametric equations for x...
-
Find the market equilibrium point for the following demand and supply functions. Demand: 2p = - q + 56 Supply: 3p - q = 34
-
Air bags are activated when a severe impact causes a steel ball to compress a spring and electrically ignite a detonator cap. This action causes sodium azide (NaN 3 ) to decompose explosively...
-
At elevated temperatures, sodium chlorate decomposes to produce sodium chloride and oxygen gas. A 0.8765-g sample of impure sodium chlorate was heated until the production of oxygen gas ceased. The...
-
Xenon and fluorine will react to form binary compounds when a mixture of these two gases is heated to 400 o C in a nickel reaction vessel. A 100.0-mL nickel container is filled with xenon and...
-
1,600 Balance Sheet The following is a list (in random order) of KIP International Products Company's December 31, 2019, balance sheet accounts: Additional Paid-In Capital on Preferred Stock $2,000...
-
Question 3 4 pts 9 x + 3 x 9 if x 0 Find a) lim f(x), b) lim, f(x), C), lim , f(x) if they exist. 3 Edit View Insert Format Tools Table : 12pt M Paragraph B IV A2 Tv
-
Mr. Geoffrey Guo had a variety of transactions during the 2019 year. Determine the total taxable capital gains included in Mr. Guo's division B income. The transactions included: 1. On January 1,...

Study smarter with the SolutionInn App


