Evaluate K c for each of the following equilibria from the value of K: (a) 2 SO(g)
Question:
Evaluate Kc for each of the following equilibria from the value of K: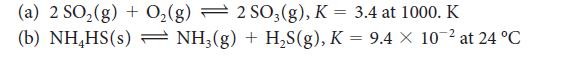
Transcribed Image Text:
(a) 2 SO₂(g) + O₂(g) 2 SO3(g), K = 3.4 at 1000. K (b) NH₂HS(s) - NH3(g) + H₂S(g), K = 9.4 x 10-² at 24 °C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Please note that the concentrations should be in molarity molL molL when substituting t...View the full answer

Answered By

Ajeet Singh
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions.
I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life.
I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge.
I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields.
Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a teacher. I have done plenty of studying and lots of learning on many exciting and challenging topics. All these experiences have influenced my decision to take on the teaching role in various capacities. As a tutor, I am looking forward to getting to understand your needs and helping you achieve your academic goals. I'm highly flexible and contactable. I am available to work on short notice since I only prefer to work with very small and select groups of students.
4.90+
7+ Reviews
15+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Evaluate K c for each of the following equilibria from the value of K: (a) 2 NOCI(g) (b) CaCO3(s) 2 NO(g) + Cl(g), K = 1.8 10- at 500 K CaO(s) + CO(g), K = 167 at 1073 K
-
Write equilibrium-constant expressions Kc for each of the following reactions. a. N203(g)NO2(8) +NO(g 2NO(g)+O2(g) 2NO2(g) PC13(g) + 3NH3(g)-P(NH2)3(g) + 3HC(g) c. d.
-
Write equilibrium-constant expressions Kc for each of the following reactions. a. N2(g) 2H2g) N2H4(g) b. 2NOCI(g) 2N0(g) + Cl2(g) c. 2NO(g) 2H2(g)N2(8) 2H20(g)
-
At the beginning of the current tennis season, on April 1, 2024, Kicked-Back Tennis Shops inventory consisted of 50 tennis racquets at a cost of $40 each. Kicked-Back uses a perpetual inventory...
-
The separation of isopentane from n-pentane by distillation is difficult (approximately 100 trays are required), but is commonly practiced in industry. Using the extended Antoine vapor pressure...
-
A vertical cylinder with a heavy piston contains air at a temperature of 300 K. The initial pressure is 200 kPa, and the initial volume is 0.350 m3. Take the molar mass of air as 28.9 g/mol and...
-
Distinguish between accounting for the employers pension plan and accounting for the pension fund.
-
Denise Nelson operates Interiors by Denise, an interior design consulting and window treatment fabrication business. Her business is made up of two different distribution channels, a consulting...
-
Suppose you make annual payments of $1000 into an account for 30 years. There is a steady return on 5% annual interest. How much money will be in the account after 30 years? Group of answer choices...
-
A farmer has 100 acres on which to plant oats or corn. Each acre of oats requires $18 capital and 2 hours of labor. Each acre of corn requires $36 capital and 6 hours of labor. Labor costs are $8 per...
-
Calculate (a) The molality of 13.63 g of sucrose, C 12 H 22 O 11 , dissolved in 612 mL of water; (b) The molality of CsCl in a 10.00% by mass aqueous solution; (c) The molality of acetone in an...
-
Use data from Table 4C.1 to calculate the vapor pressure of mercury at 275 K. TABLE 4C.1 Standard Enthalpies of Physical Change* Freezing AH fus% (kJ. mol) point, T/K Substance Formula acetone...
-
Owen has just received an inheritance of $20,000. He has deposited the funds into an account that will earn 5% compounded quarterly. Owen plans to add to his savings by making end-of month deposits...
-
Each of the accompanying graphs shows a do plot of data from three separate random samples for each of the four graphs, indicate whether you think that the basic assumptions for single-factor ANOVA...
-
Program Milestones Milestone #1 - Selection GUI - Create Account/Login/Cancel Obtain a copy of Eclipse and complete "Getting Started in Eclipse". Create your project in Eclipse and the package and...
-
= The momentum transfer is q = ph - Ph, where p is the hadron momentum after the collision. This relationship holds for the time as well as the space components, i.e., for the 4-vectors. Thus, we...
-
When should HR be the interviewer, and when should a hiring manager or co-workers be involved? Should reference checking be done before or after the interview? Would you ask during the interview any...
-
1. Monoclean Company manufactures a single product, Glamour. The standard cost specification sheet shows the following standards for one unit of Glamour: 8 kg of material M @ $6.5 per kg $52 4 hours...
-
Why are workers in accounting, operations, sales, executive/upper management, customer service, and purchasing functions most likely to commit fraud?
-
Describe the general ways that the revised Form 990, applicable for tax year 2008 and beyond, is different from previous versions.
-
Draw all resonance structures for each of the following radicals: (a) (b) (c) (d) (e)
-
Why is the standard state of fugacity, f , equal to the standard state of pressure, P?
-
A compressed cylinder of gas contains 2.74 10 3 g of N 2 gas at a pressure of 3.75 10 7 Pa and a temperature of 18.7C. What volume of gas has been released into the atmosphere if the final pressure...
-
You have $55,000. You put 15% of your money in a stock with an expected return of 10%, $38,000 in a stock with an expected return of 18%, and the rest in a stock with an expected return of 22%. What...
-
Portfolio return and beta Personal Finance Problem Jamie Peters invested $ 1 1 3 , 0 0 0 to set up the following portfolio one year ago: a . Calculate the portfolio beta on the basis of the original...
-
. Emerson Cammack wishes to purchase an annuity contract that will pay him $7,000 a year for the rest of his life. The Philo Life Insurance Company figures that his life expectancy is 20 years, based...

Study smarter with the SolutionInn App


