Evaluate K c for each of the following equilibria from the value of K: (a) 2 NOCI(g)
Question:
Evaluate Kc for each of the following equilibria from the value of K: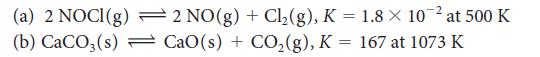
Transcribed Image Text:
(a) 2 NOCI(g) (b) CaCO3(s) 2 NO(g) + Cl₂(g), K = 1.8 × 10-² at 500 K CaO(s) + CO₂(g), K = 167 at 1073 K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a K c...View the full answer

Answered By

Shadrack Mulunga
I am a Biochemistry by profession. However, I have explored different fields of study. My quest to explore new fields has helped me gain new knowledge and skills in Business, clinical psychology, sociology, organizational behavior and general management, and Project Management. I count my expertise in Project management, in particular, creation of Work Break Down Structure (WBS) and use of Microsoft Project software as one of my greatest achievement in Freelancing industry. I have helped thousands of BSC and MSC students to complete their projects on time and cost-effectively using the MS Project tool. Generally, I find happiness in translating my knowledge and expertise to success of my clients. So far, i have helped thousands of students to not only complete their projects in time but also receive high grades in their respective courses. Quality and timely delivery are the two key aspects that define my work. All those who hired my services always come back for my service. If you hire my services today, you will surely return for more. Try me today!
5.00+
154+ Reviews
289+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Evaluate K c for each of the following equilibria from the value of K: (a) 2 SO(g) + O(g) 2 SO3(g), K = 3.4 at 1000. K (b) NHHS(s) - NH3(g) + HS(g), K = 9.4 x 10- at 24 C
-
Write equilibrium-constant expressions Kc for each of the following reactions. a. N203(g)NO2(8) +NO(g 2NO(g)+O2(g) 2NO2(g) PC13(g) + 3NH3(g)-P(NH2)3(g) + 3HC(g) c. d.
-
Write equilibrium-constant expressions Kc for each of the following reactions. a. N2(g) 2H2g) N2H4(g) b. 2NOCI(g) 2N0(g) + Cl2(g) c. 2NO(g) 2H2(g)N2(8) 2H20(g)
-
Which of the following variables was controlled in Experiment 1? F. Amount of yeast G. Percent of molasses H. Percent of sucrose J. Carbon dioxide levels Experiment 1 Since yeast needs sucrose to...
-
Explain why the separation of a stream containing 10 wt% acetic acid in water might be more economical by liquid-liquid extraction with ethyl acetate than by distillation.
-
Discuss three common examples of natural processes that involve an increase in entropy. Be sure to account for all parts of each system under consideration.
-
What do the numbers mean? General Electrics 2001 annual report is 93 pages and has 30 percent more financial information than the year before. Primarily GE provided more specific data about 26...
-
The 2011 balance sheet of Annas Tennis Shop, Inc., showed $490,000 in the common stock account and $3.4 million in the additional paid-in surplus account. The 2012 balance sheet showed $525,000 and...
-
ABC company had net sales of $40,000 and accounts receivable of $4,800. Its days sales uncollected is: 36.5 days. 35.04 days. 40.15 days. 29.20 days. 43.80 days.
-
On May 1, a petty cash fund was established for $150. The following vouchers were issued during May: Date Voucher No. Purpose Amount May 1 1 postage due $ 3.50 3 2 office supplies 11.00 5 3 auto...
-
State what happens to the concentration of the indicated substance when the total pressure on each of the following equilibria is increased (by compression): (a) NO(g) in 2 Pb (NO3)2(s) 2 PbO (s) + 4...
-
The density of a 5.00% by mass K 3 PO 4 aqueous solution is 1.043 g cm 3 . Determine (a) The molality; (b) The molarity of potassium phosphate in the solution.
-
The Navy wanted to conduct training exercises off the coast of California for sonar submarines. Scientists were concerned that the sounds emitted by the sonar would harm marine mammals, such as...
-
Question 4 25 p J Mart is considering purchasing a new inventory control system featuring state-of-the-art technology. Two vendors have submitted proposals to supply J Mart with the new system. The...
-
ME 2352 Design Optimization Assignment TWO, due February 6th, 2024, 4:00 pm University of New Brunswick Department of Mechanical Engineering 1. By use of definition of linear dependency determine if:...
-
IKEA's People and Planet Positive sustainability plan, launched in 2012, aims to contribute to a better life for people and a better future for the planet. The plan outlines several sustainable goals...
-
Question 4 [25 marks] A cantilever beam AB is fixed to a wall and is subjected to concentrated and distributed loads as shown in figure B1. a) Draw the free-body diagram of the problem. [5 marks] a)...
-
GMC is an Australian farm machinery manufacturer, operating since 1975. The company makes high-quality farm machinery and equipment including a range of slashers, mowers, aerators, mulchers and...
-
Can hackers tell that you have a honeypot running?
-
The vapor pressure of the liquid NH, is measured at different temperatures. The following vapor pressure data are obtained. Temperature, K P, mmHg 217.1 223.4 234.7 588.1 Calculate the enthalpy of...
-
Acid-catalyzed hydration of 1-methylcyclohexene yields two alcohols. The major product does not undergo oxidation, while the minor product will undergo oxidation. Explane.
-
Calculate S surroundings and S total for part (c) of Problem P5.6. Is the process spontaneous? The state of the surroundings is T = 310.K, P = 0.333 bar.
-
Acid-catalyzed hydration of 1-methylcyclohexene yields two alcohols. The major product does not undergo oxidation, while the minor product will undergo oxidation. Explane.
-
On April 1, year 1, Mary borrowed $200,000 to refinance the original mortgage on her principal residence. Mary paid 3 points to reduce her interest rate from 6 percent to 5 percent. The loan is for a...
-
Give a numerical example of: A) Current liabilities. B) Long-term liabilities?
-
Question Wonder Works Pte Ltd ( ' WW ' ) produces ceramic hair curlers to sell to department stores. The production equipment costs WW $ 7 0 , 0 0 0 four years ago. Currently, the net book value...

Study smarter with the SolutionInn App


