State what happens to the concentration of the indicated substance when the total pressure on each of
Question:
State what happens to the concentration of the indicated substance when the total pressure on each of the following equilibria is increased (by compression):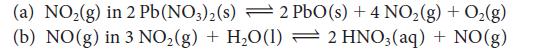
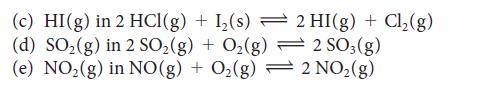
Transcribed Image Text:
(a) NO₂(g) in 2 Pb (NO3)2(s) 2 PbO (s) + 4 NO₂ (g) + O₂(g) 2 HNO3(aq) + NO(g) (b) NO(g) in 3 NO₂(g) + H₂O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a In the reaction 2 PbNO32s 2 PbOs 4 NO2g O2g increasing the total pressure by compression will shif...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
When we double the compression ratio of an ideal Otto cycle, what happens to the maximum gas temperature and pressure when the state of the air at the beginning of the compression and the amount of...
-
Consider the equilibrium CO(g) + H 2 O(g) CO 2 (g) + H 2 (g). (a) If the partial pressure of CO 2 is increased, what happens to the partial pressure of H 2 ? (b) If the partial pressure of CO is...
-
Each of the following three data sets represents the IQ scores of a random sample of adults. IQ scores are known to have a mean and median of 100. For each data set, determine the sample standard...
-
Why is the number of equivalent units for materials only sometimes equal to the equivalent units for conversion?
-
A hydrocarbon stream in a petroleum refinery is to be separated at 1,500 kPa into two products under the conditions shown below. Using the data given, compute the minimum work of separation, Wmin ,...
-
An idealized diesel engine operates in a cycle known as the air-standard diesel cycle, shown in Figure 22.14. Fuel is sprayed into the cylinder at the point of maximum compression; B. Combustion...
-
(EPS: Simple Capital Structure) A portion of the combined statement of income and retained earnings of Seminole Inc. for the current year follows. At the end of the current year, Seminole Inc. has...
-
Klein Sisters Products Inc. manufactures a liquid product in one department. Due to the nature of the product and the process, units are regularly lost at the beginning of production. Materials and...
-
Suppose a firm imports goods from Europe and the import price is denominated in euros, then ________. Group of answer choices the exporter bears foreign exchange risk Central Bank faces foreign...
-
On May 1, a petty cash fund was established for $150.00. The following vouchers were issued during May: REQUIRED 1. Prepare the journal entry to establish the petty cash fund. 2. Record the vouchers...
-
Two unknown compounds were being studied. Compound C is molecular and compound D is an ionic compound known to dissociate into ions completely in dilute aqueous solutions. A solution containing 0.30...
-
Evaluate K c for each of the following equilibria from the value of K: (a) 2 NOCI(g) (b) CaCO3(s) 2 NO(g) + Cl(g), K = 1.8 10- at 500 K CaO(s) + CO(g), K = 167 at 1073 K
-
The following T account is a summary of the cash account of Edmonds Company. What amount of net cash provided (used) by financing activities should be reported in the statement of cashflows? Cash...
-
Salmone Company reported the following purchases and sales of its only product. Salmone uses a perpetual inventory system. Determine the cost assigned to the ending inventory using FIFO. 1 Date...
-
A company may go through organizational change at various stages in its life cycle for a variety of reasons. Reasons can include a change in ownership as well as a change in the competitive...
-
6 (a) Below is a diagram of a rotating disc viscometer (FIGURE 4). Explain its operations and limitations as to use. If, in a similar works situation, it is necessary to make measurements on a...
-
As part of your role in the Business Analytics and Data Analytics team, you have been asked to forecast Food Retailing as part of a wider report being commissioned by the above collaboration - on...
-
You are three students who have together bought a business that makes snow. The customers consist of both large public enterprises and private individuals. The business is run all year round, but the...
-
Do they have honeypots for spammers to keep them from harvesting e-mails from your webpages?
-
This problem continues the Draper Consulting, Inc., situation from Problem 12-45 of Chapter 12. In October, Draper has the following transactions related to its common shares: Oct 1 Draper...
-
A 1.25 mole sample of an ideal gas is expanded from 320. K and an initial pressure of 3.10 bar to a final pressure of 1.00 bar, and C P,m = 5/2R. Calculate w for the following two cases: a. The...
-
What reagents would you use to perform each of the following transformations? a. b. H.
-
Propose a mechanism for the following reaction. NaH Br
-
A company is evaluating a new 4-year project. The equipment necessary for the project will cost $3,300,000 and can be sold for $650,000 at the end of the project. The asset is in the 5-year MACRS...
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
I need to see where the calculations for this problem come from plz. 5. Award: 4.00 points Lucido Products markets two computer games: Claimjumper and Makeover. A contribution format income statement...

Study smarter with the SolutionInn App


