For each of the following alcohols, give the systematic name, and specify whether the alcohol is primary,
Question:
For each of the following alcohols, give the systematic name, and specify
whether the alcohol is primary, secondary, or tertiary.
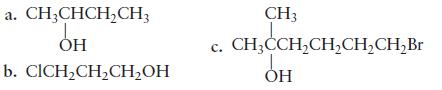
Transcribed Image Text:
a. CH3CHCHCH3 b. CICHCHCHOH . CH3 CH3CCH, CH,CH, CH,Br
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a The chain is numbered as follows 1 2 3 4 CH3CHCHCH3 OH The compoun...View the full answer

Answered By

Talha Talib
I am a member of IEEE society. As i am a student of electrical engineering badge 17 but beside of this i am also a tutor in unique academy. I teach calculus, communication skills, mechanics and economics. I am also a home tutor. My student Muhammad Salman Alvi is a brilliant A-level student and he performs very well in academics when i start to teach him. His weak point was mathematics but now he is performing well in mathematics. I am a scholarship holder in Fsc as i scored 1017 marks in metric out of 1100. Later on i got scholarship in Punjab Group of Colleges. I got 2nd position in robotics competition in 2018 as my project home automation select for the exhibition in Expocentre.
4.60+
23+ Reviews
62+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For each of the following alcohols, give the systematic name and specif) whether the alcohol is primary, sec-ondary, or tertiary. a. b. c. Cl CH:CHCH2CH2 CH2CH2CH, CH3CCH2CH3 CH3 OH
-
Write structural formulas for each of the following: (a) Three ethers with the formula C4H10O. (b) Three primary alcohols with the formula C4H8O. (c) A secondary alcohol with the formula C3H6O. (d) A...
-
Give an IUPAC systematic or common name for each of the following compounds: (a) (b) (c) (d) (e) (f) (g) (h) CH3CN Cl NH2
-
If you were a hedge fund manager, which style would you employ and why? Describe this style and how it works. Why would you utilize it and under what macroeconomic conditions would this style work...
-
Use properties of integrals, together with Exercises 27 and 28, to prove the inequality. 26 + I dx 61. r sin rdx s 8.
-
Following are data related to a product of Coen Company for the year 1999: a. Assuming use of perpetual inventory procedure, compute the ending inventory and cost of goods sold under each of the...
-
Explain the classification of cost in detail.
-
Part of your companys a c counting database was destroyed when Godzilla attacked the city. You have been able to gather the following data from your files. Reconstruct the remaining information using...
-
I cant figure out the the 2017 return on common stockholders equity and the debt to assests ratio Question 9 0. Blossom Company has $ 1,000,000 in assets and $1,000,000 in stockholders' equity, with...
-
Write the sequences of all possible tripeptides composed of the amino acids tyrosine, histidine, and cysteine.
-
Name each of the following molecules. (a) (b) H CH3CHCH CH3 C=C CH3 H
-
Find an equation for the plane tangent to the surface z = (x, y) at the given point. z = 1/(x 2 + y 2 ), (1, 1, 1/2)
-
What can you do to plan ahead and educate others about the international groups? Consider How do you communicate during the meeting with your colleagues?
-
What do you think should be the role of personality tests in candidate selection? Do you think they should play a major, minor or no part in an organization\'s hiring decision for a job? What are...
-
What channels do our target customers prefer for discovering, researching, and purchasing products? How do cultural or societal shifts affect consumer attitudes and behaviors towards our products or...
-
What role do symbolic artifacts and rituals play in the construction and maintenance of organizational culture, and how do they influence employee identification and commitment ?
-
Describe all you would do and what you would consider in converting some or all of your employees to independent contractors, the rate you would pay and the reason therefor and how it would be paid....
-
Consider a declining population following the formula b(t) = l / 1 + t (measured in millions). Approximate the population at each of the following times using the tangent with base point t = 0, the...
-
President Lee Coone has asked you to continue planning for an integrated corporate NDAS network. Ultimately, this network will link all the offices with the Tampa head office and become the...
-
Consider the equilibrium A (g) 2 B (g) + 3 C (g) at 25C. When A is loaded into a cylinder at 10.0 atm and the system is allowed to come to equilibrium, the final pressure is found to be 20.04 atm....
-
Using data from Table 5G.2 and standard graphing software, determine the standard enthalpy and entropy of the reaction N 2 O 4 (g) 2 NO 2 (g) and estimate the NN bond enthalpy in N 2 O 4 . How does...
-
The three compounds methylpropene, cis-2-butene, and trans-2-butene are isomers with the formula C 4 H 8 , with G f 5 +58.07, +65.86, and +62.97 kJ mol 1 , respectively. In the presence of a...
-
please help Problem 13-7 (Algo) Prepare a Statement of Cash Flows [LO13-1, LO13-2] [The following information applies to the questions displayed below.] Comparative financial statements for Weaver...
-
A firm has 1000 shareholders, each of whom own $59 in shares. The firm uses $28000 to repurchase shares. What percentage of the firm did each of the remaining shareholders own before the repurchase,...
-
Vancouver Bank agrees to lend $ 180,000 to Surrey Corp. on November 1, 2020 and the company signs a six-month, 6% note maturing on May 1, 2021. Surrey Corp. follows IFRS and has a December 31 fiscal...

Study smarter with the SolutionInn App


