For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or
Question:
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: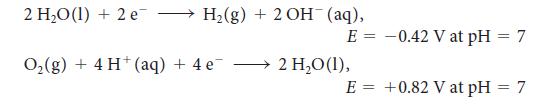
(a) When a current of 324 mA is used for 15 h, what volume (measured in liters at 298 K and 1.0 atm) of fluorine gas can be produced from a molten mixture of potassium and hydrogen fluorides?
(b) With the same current and time period, how many liters of oxygen gas at 298 K and 1.0 atm can be produced from the electrolysis of water?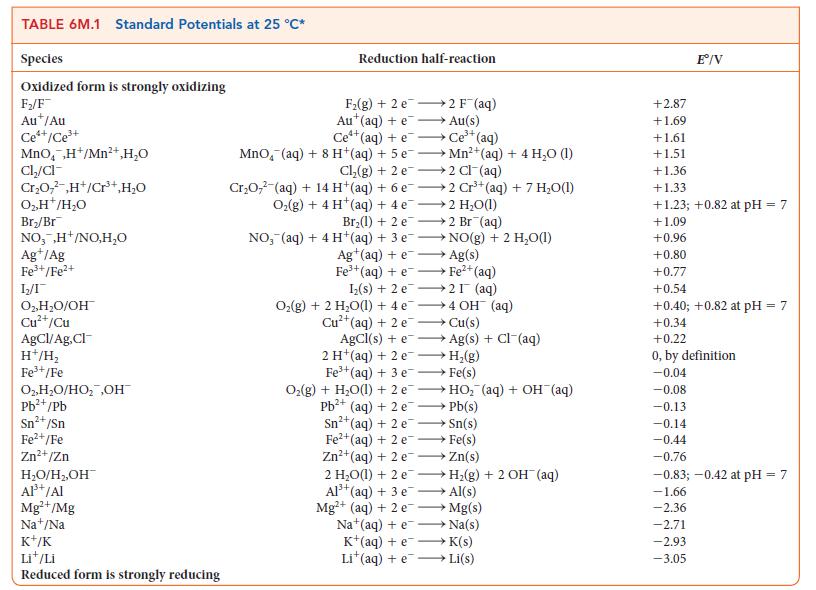
Transcribed Image Text:
2 H₂O (1) + 2 e → H₂(g) + 2OH(aq), O₂(g) + 4 H(aq) + 4e¯ E = -0.42 V at pH = 7 2 H₂O (1), E = +0.82 V at pH = 7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
To find the volumes of fluorine gas and oxygen gas produced through electrolysis based on a given cu...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: Thomas Edison was faced...
-
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: A total charge of 4.5 kC...
-
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: A total charge of 67.2...
-
Question Description RangeFilterTester.java import java.util.ArrayList; class RangeFilterTester { public static void main( String[] args) { ArrayList accounts = new ArrayList(); accounts.add(new...
-
Single-rate versus dual-rate methods, support department. The Chicago power plant that services all manufacturing departments of MidWest Engineering has a budget for the coming year. This budget been...
-
Past data indicate that, the amount of money contributed by the working residents of a large city to a volunteer rescue squad is a normal random variable with a standard deviation of S1.40. It has...
-
What strategy can a project manager use to deliver bad news? a. Tell a joke first. b. Tell senior management as soon as possible so they can develop alternatives and recommendations. c. Ask the...
-
From the 1960s, James Johnson served as Bradley Unions personal caretaker and assistant, and was authorized by Union to handle his banking transactions. Louise Johnson, Jamess wife, wrote checks on...
-
As of the end of the period, the value added tax balance of the business to be deducted is 8000 TL; 391 calculated value added tax account balance is 6000 TL. What is the closing registration to be...
-
Complete Form SS-4 for TCLH Industries. The company was formed on December 1, 2019, as a corporation (which files Form 1120S and was incorporated in North Carolina) by Michael Sierra (CEO; SSN 232-...
-
The percentage deprotonation of benzoic acid in a 0.110 m solution is 2.4%. What is the pH of the solution and the Ka of benzoic acid?
-
A tin electrode in 0.015 m Sn(NO 3 ) 2 (aq) is connected to a hydrogen electrode in which the pressure of H 2 is 1.0 bar. If the cell potential is 0.061 V at 25C, what is the pH of the electrolyte at...
-
How can consumers use the Internet to help control the ethical behavior of business leaders?
-
The ratio of CEO pay to that of an average employee increased over a period of 50 years from 24:1 to 275:1. Is this increasing gap ethically sound, in your opinion? Should CEO pay be limited in any...
-
Suppose you are considering buying a machine that costs $7,000. It will generate revenues of $1,500 for the next 3 years, and then $1,000 for the following 5 years. What is the payback period of this...
-
National Bakery Limited is the main supplier of a variety of baked products to customers in Kingston. The company currently makes 25,000,000 a variety of baked products annually which uses baking...
-
Q1. Discuss the financial goal of a business. Ensure to provide an example of the inherent ethical challenges associated with the financial goal and or the financial management process. Using the...
-
Q1. How can companies use social media to do sentiment analysis? Describe the process. Give an example of a company that uses sentiment analysis to enhance relationships with customers. Q2. Describe...
-
Consider random sampling from a dichotomous population with p = 0.3, and let E be the event that is within 0.05 of p. Use the normal approximation (without the continuity correction) to calculate...
-
Provide a draft/outline of legal research involving an indigenous Canadian woman charged with assault causing bodily harm under (Sec 267b) of the Criminal Code, where the crown wants a 12-month jail...
-
Calculate I, , and a for a 0.0215 m solution of K 2 SO 4 at 298 K. How confident are you that your calculated results will agree with experimental results?
-
Calculate I, , and a for a 0.0175 m solution of Na 3 PO 4 at 298 K. Assume complete dissociation. How confident are you that your calculated results will agree with experimental results?
-
Calculate the ionic strength of each of the solutions in Problem P10.4. In Problem V-l, + V-H- Usolute
-
business law A partner may actively compete with the partnership True False
-
A company provided the following data: Selling price per unit $80 Variable cost per unit $45 Total fixed costs $490,000 How many units must be sold to earn a profit of $122,500?
-
Suppose a 10-year, 10%, semiannual coupon bond with a par value of $1,000 is currently selling for $1,365.20, producing a nominal yield to maturity of 7.5%. However, it can be called after 4 years...

Study smarter with the SolutionInn App


