For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or
Question:
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: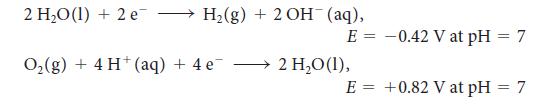 Thomas Edison was faced with the problem of measuring the electricity that each of his customers had used. His first solution was to use a zinc “coulometer,” an electrolytic cell in which the quantity of electricity is determined by measuring the mass of zinc deposited. Only some of the current used by the customer passed through the coulometer.
Thomas Edison was faced with the problem of measuring the electricity that each of his customers had used. His first solution was to use a zinc “coulometer,” an electrolytic cell in which the quantity of electricity is determined by measuring the mass of zinc deposited. Only some of the current used by the customer passed through the coulometer.
(a) What mass of zinc would be deposited in 1 month (of 31 days) if 1.0 mA of current passed through the cell continuously?
(b) An alternative solution to this problem is to collect the hydrogen produced by electrolysis and measure its volume. What volume would be collected at 298 K and 1.00 bar under the same conditions?
(c) Which method would be more practical?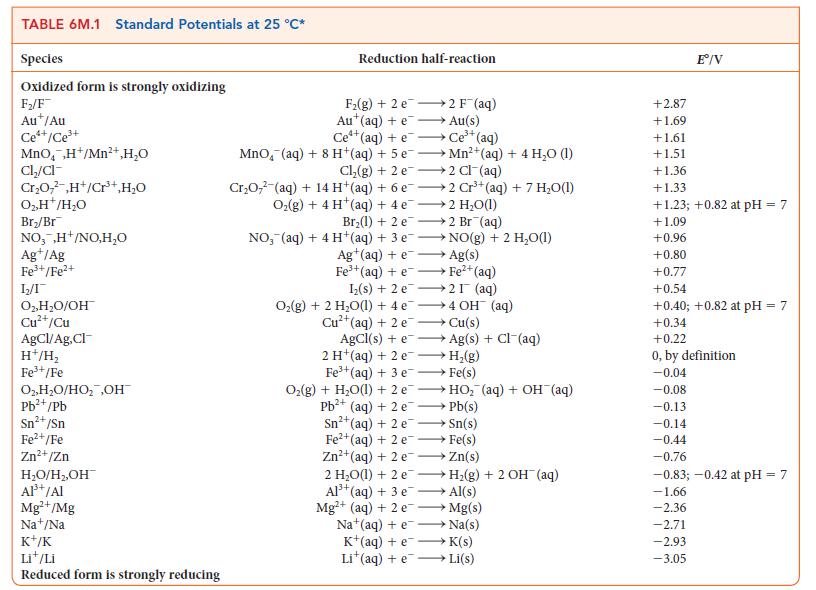
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





