For the reaction 2NO5(g) 4NO2(g) + O(g)
Question:
For the reaction![]()
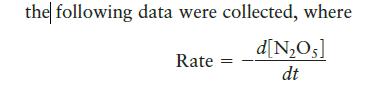
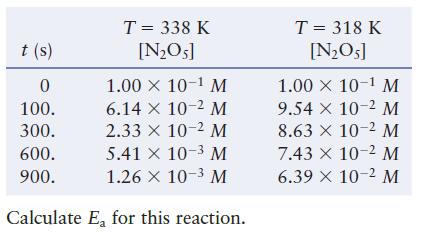
Transcribed Image Text:
2N₂O5(g) 4NO2(g) + O₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (12 reviews)
The given reaction is represented below 2NO g NO g O g 1 Taking NO at 0 and 100 secon...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For the endothermic reaction the following represents a reaction container at two different temperatures. Which one (I or II) is at the lower temperature? AB(g) A(g) B(g),
-
For the following reaction profiles, indicate The positions of reactants and products. The activation energy. ÎE for the reaction. The second reaction profile is representative of a reaction...
-
For each of the following reaction coordinate diagrams, tell whether the structure of the transition state will more closely resemble the structure of the reactants or the structure of the products:...
-
Suppose a town concludes that it costs on average $30.00 per household to manage the disposal of the waste generated by households each year. It is debating two strategies for funding this cost: (1)...
-
A vending machine dispenses coffee into an eight-ounce cup. The amounts of coffee dispensed into the cup are normally distributed, with a standard deviation of 0.03 ounce. You can allow the cup to...
-
Multiplication and division. Simplify. 2a(-3b)(-4c)(-1)
-
Bird colonies Refer to your graph from Exercise 6. (a) Describe the relationship between number of new sparrowhawks in a colony and percent of returning adults. (b) For short-lived birds, the...
-
Depreciation information for Corales Company is given in BE10-3. Assuming the declining-balance depreciation rate is double the straight-line rate, compute annual depreciation for the first and...
-
Accounting for pensions receives more attention in the United States than in other countries. Discuss reasons that would explain why pension accounting has less emphasis in many foreign countries...
-
You work in the human resources department of your company helping new employees fill out the necessary paperwork to get their first paycheck. There are a number of decisions that employees must make...
-
Which of the changes listed below would affect the value of the rate constant k? a. Increasing the partial pressure of oxygen gas. b. Changing the temperature. c. Using an appropriate catalyst. - For...
-
For the reaction A + B C, explain at least two ways in which the rate law could be zero order in chemical A.
-
Use the Intermediate Value Theorem to verify that the following equations have three solutions on the given interval. Use a graphing utility to find the approximate roots. 70x 3 - 87x 2 + 32x - 3 =...
-
Determine the magnitude of the magnetic flux through the south-facing window of a house in British Columbia, where Earth's B field has a magnitude of 5.8 x 10-5T and the direction of B field is 72...
-
A wedge with an inclination of angle rests next to a wall. A block of mass m is sliding down the plane, as shown. There is no friction between the wedge and the block or between the wedge and the...
-
Conner Leonard worked for Purges Manufacturing for 32 years. Along with four other men, he helped to start the company that designed and built products sold around the world. Purges Manufacturing...
-
Reconsider the collision between two objects diagrammed below where two objects move on a frictionless surface. Before collision After collision Experiment 1 A, 1 B A B Draw complete and properly...
-
3. Now the bomb arrives. Please catch fx,y(x, y) = = cx cx - dy, where 0 < x < 1, 0 y x. 13 a) Please find coefficients c, d such that cd= 8 b) Please find fx(x) and fy (y). Are X and Y independent?...
-
Find the slope-intercept form of the equation of the line passing through the point (-6, 4) and perpendicular to the graph of 3x - 2y = 6.
-
A crop-dusting plane flies over a level field at a height of 25 ft. If the dust leaves the plane through a 30 angle and hits the ground after the plane travels 75 ft, how wide a strip is dusted? See...
-
Structural isomers are compounds that have the same chemical formula but the atoms are bonded together differently giving different compounds. Consider the two structural isomers having the formula...
-
The sp2 hybrid atomic orbitals have the following general form: where Ïs, Ïpx, and Ïpy represent orthonormal (normalized and orthogonalized) atomic orbitals. Calculate the values of A...
-
Experimental values for the temperature dependence of the rate constant for the gas-phase reaction NO(g) + O3(g) NO2(g) + O2(g) are as follows: Make the appropriate graph using these data, and...
-
You plan to buy a house for $325,000 today. If the house is expected to appreciate in value 8% each year, what will its value be seven years from now?
-
A designated beneficiary of an ABLE account must be ___________ in order to meet the special rules that apply to the increased contribution limit authorized under the Tax Cuts and Jobs Act? a. an...
-
Stans wholesale buys canned tomatoes from canneries and sells them to retail markets Stan uses the perpetual inventory

Study smarter with the SolutionInn App


