Which of the changes listed below would affect the value of the rate constant k? a. Increasing
Question:
Which of the changes listed below would affect the value of the rate constant k?
a. Increasing the partial pressure of oxygen gas.
b. Changing the temperature.
c. Using an appropriate catalyst.
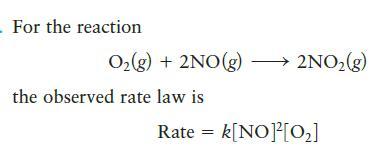
Transcribed Image Text:
- For the reaction O₂(g) + 2NO(g) →→→→ 2NO₂(g) the observed rate law is Rate = k[NO]²[0₂]
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
Will the rate constant change if a Increasing the partial pressure of ...View the full answer

Answered By

Jessica Souza
I work as Subject matter expert in Science and Medicine for some local firms. Besides I teach the underpriveleged kids with some NGOs. I am very passionate to spread knowledge.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which of the changes being considered by the manager of Cheng Parts Company go counter to the JIT philosophy?
-
What effect will each of the changes listed in Study Question of Chapter 27 have on the equilibrium level of GDP in the private closed economy? Explain your answers. a. A large increase in the value...
-
An ideal gas initially at temperature To, pressure po, and volume Vo is compressed to one-half its initial volume. As shown in Fig. 12.20, process 1 is adiabatic, 2 is isothermal, and 3 is isobaric....
-
What pricing system is used to price the water you use at your college or university? Does this pricing system affect your behavior about water use (length of showers, etc.)? How? Could you recommend...
-
In a large section of a statistics class, the points for the final exam are normally distributed, with a mean of 72 and a standard deviation of 9. Grades are assigned according to the following rule....
-
Simplify. (-4) 3 x (-4)
-
Does fast driving waste fuel? How does the fuel consumption of a car change as its speed increases? Here are data for a British Ford Escort. Speed is measured in kilometers per hour, and fuel...
-
Explain why a state has regulatory authority over a not-for-profit organization. When does the federal government have regulatory authority over a not-for-profit organization?
-
Car, Home, Retirement: What s My Plan? Looking at her schedule, Jocelyn realized that she only had a few hours before her first meeting with her financial advisor. She had started her first job in...
-
1. Given the risk, what would motivate an investor to purchase stock in Gogo? 2. Why would Gogo sell stock rather than taking on additional debt financing? Do you think that this was a good decision?...
-
The thiosulfate ion (S 2 O 3 2- ) is oxidized by iodine as follows: In a certain experiment, 7.05 10 -3 mol/L of S 2 O 3 2- is consumed in the first 11.0 seconds of the reaction. Calculate the rate...
-
For the reaction 2NO5(g) 4NO2(g) + O(g)
-
What is the process of asset transformation performed by a financial institution? Why does this process often lead to the creation of interest rate risk? What is interest rate risk?
-
7. A psychiatrist is testing a new ADHD Medication, which seems to have the potentially harmful side effect of increasing the heart rate. For a sample of 50 clinical study participants whose pulse...
-
Determine the type of engagement that your colleague completed for the client. Justify the selected engagement type for the client. Assess the purpose of each financial statement for the client's...
-
Mills Corporation acquired as a long-term investment $235 million of 8% bonds, dated July 1, on July 1, 2024. Company management has classified the bonds as an available-for-sale investment. The...
-
A force of 28 pounds acts on the pipe wrench shown in the figure below. 18 in. 30 (a) Find the magnitude of the moment about O by evaluating ||OA x F||. (0 0 180) Use a graphing utility to graph the...
-
Module 1 1. There has been a rise in cases of measles in RI. The RI Health Department is wondering if the rate of MMR vaccinations has declined since the start of the COVID-19 pandemic. The...
-
Fill in the blank to correctly complete each sentence. The point (4, _____) lies on the graph of the equation y = 3x - 6.
-
Determine which of the following limits exist. Compute the limits that exist. lim x-0 1- + 3x X
-
When isopropylbenzene (cumene) is treated with NBS and irradiated with UV light, only one product is obtained. Propose a mechanism, and explain why only one product is formed. Br NBS hv
-
Assign an IUPAC name for each of the following compounds: a. b. c. d. .
-
Given the data in Table 4.1 (Appendix B, Data Tables) and the following information, calculate the single bond enthalpies and energies for SiF, SiCl, CF, NF, OF, HF: HF(g) SiF,(g) SiCl,(g) CF,(g)...
-
On April 1, year 1, Mary borrowed $200,000 to refinance the original mortgage on her principal residence. Mary paid 3 points to reduce her interest rate from 6 percent to 5 percent. The loan is for a...
-
Give a numerical example of: A) Current liabilities. B) Long-term liabilities?
-
Question Wonder Works Pte Ltd ( ' WW ' ) produces ceramic hair curlers to sell to department stores. The production equipment costs WW $ 7 0 , 0 0 0 four years ago. Currently, the net book value...

Study smarter with the SolutionInn App


