Question: Given the data in Exercise 87 on substance X, calculate the energy that must be removed to convert 250 . g of substance (mathrm{X}) from
Given the data in Exercise 87 on substance X, calculate the energy that must be removed to convert 250 . g of substance \(\mathrm{X}\) from a gas at \(100 .{ }^{\circ} \mathrm{C}\) to a solid at \(-50 .{ }^{\circ} \mathrm{C}\). Assume \(\mathrm{X}\) has a molar mass of \(75.0 \mathrm{~g} / \mathrm{mol}\).
Data from Exercises 87
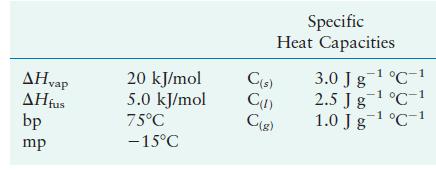
A substance has the following properties:
Sketch a heating curve for the substance, starting at \(-50^{\circ} \mathrm{C}\).
AHvap AHfus bp mp 20 kJ/mol 5.0 kJ/mol 75C -15C C(s) Specific Heat Capacities C(1) C(g) -1 3.0 J g C 1 2.5 Jg- C-1 1.0 J g C 1
Step by Step Solution
3.46 Rating (156 Votes )
There are 3 Steps involved in it
In order to calculate the energy that must be removed to convert 250 g of substance X from a gas at 100 C to a solid at 50 C well need to account for ... View full answer

Get step-by-step solutions from verified subject matter experts


