Identify the arrangement (I, II, or III; all molecules are NH 3 ) that should possess the
Question:
Identify the arrangement (I, II, or III; all molecules are NH3) that should possess the strongest intermolecular attractions, and justify your selection.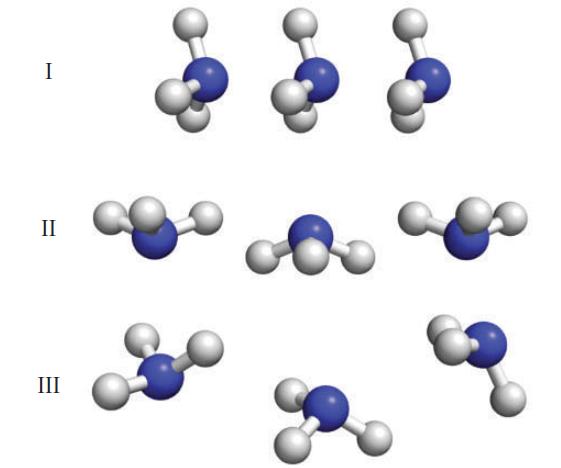
Transcribed Image Text:
I II III
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
Selection I shows the weakest intermolecular attractions because all t...View the full answer

Answered By

Khurram shahzad
I am an experienced tutor and have more than 7 years’ experience in the field of tutoring. My areas of expertise are Technology, statistics tasks I also tutor in Social Sciences, Humanities, Marketing, Project Management, Geology, Earth Sciences, Life Sciences, Computer Sciences, Physics, Psychology, Law Engineering, Media Studies, IR and many others.
I have been writing blogs, Tech news article, and listicles for American and UK based websites.
4.90+
5+ Reviews
17+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Identify the arrangement (I, II, or III; all molecules are CH 2 Cl 2 ) that should possess the strongest intermolecular attractions, and justify your selection. H II III
-
Champion Stores are in trouble. Although the company is a major regional player with hundreds of stores in the upper Midwest, a sharp decline in the regions manufacturing economy has put the company...
-
Suppose the relationship between wage, years of education (educ), years of experience (exper), and participation in a job training program (train) is modeled as: log ( ) = + + + . Which of the...
-
A firm has $180 million in annual sales; $40 million of inventory and $60 million of accounts receivable. What is the inventory turnover ratio?
-
Determine the direction ? for 0? ? ? ? 180 of the force F so that it produces the maximum moment about point A. Calculate this moment. F= 400 N 2 m -3 m-
-
Kerry and Danielle wanted to investigate whether tapping on a can of soda would reduce the amount of soda expelled after the can has been shaken. For their experiment, they vigorously shook 40 cans...
-
How did government intervention evolve between the first and second halves of the twentieth century? LO.1
-
1. Who, if anyone, suffers when some workers get flexible hours? What would be a fair way to distribute the costs and benefits of flexibility in work schedules? 2. Do employee benefits have to be...
-
MSI is considering outsourcing the production of the handheld control module used with some of its products. The company has received a bid from Monte Legend Co. (MLC) to produce 23,000 units of the...
-
The density of molybdenum is 10.22 g cm 3 and its atomic radius is 136 pm. Is the metal close-packed or body-centered cubic?
-
Show that the van der Waals parameter b is related to the molecular volume V mol , the volume occupied by one molecule, by b = 4N A V mol . Treat the molecules as spheres of radius r, so that V mol =...
-
Read the following scenario: Alex Grant recently graduated from university and is excited to be starting his first job as a store manager for The Grocery Cart, a large supermarket chain. The company...
-
Consider: x3 + c (x 1)(x 3)(x + 1) (x + 3x + 9) (x + 2x + 5) How many partial fractions are there in the partial fraction decomposition of this function? How many unknowns (A, B, ...) must be...
-
Hand trace the following program. 1 y 0 2 for x in range (5): y = y + x 4 print ("x",x, "and y =", y) Note: You can shorten the prompts in your hand trace if you want to.
-
Determine the location using physics calculations to solve the problem. Show step by step details for how you solved the problem. I don't need an explanation explaining how to solve the problem. T By...
-
Give a brief explanation about the organization/company i.e., the products or services, number of employees, etc. Do a SWOT chart to help organize your ideas. Refer to resources in the reading for an...
-
How are organization "formal" and "informal" structures impacted in organizational change? Provide some examples. Compare and contrast Lewin's Change Model with Kotter's Change model. (Show how they...
-
When a beam of light passes through a polarized lens, its intensity is cut in half, or I1 = 0.5I0. To further reduce the intensity, you can place another polarized lens in front of it. The intensity...
-
Imagine a sound wave with a frequency of 1.10 kHz propagating with a speed of 330 m/s. Determine the phase difference in radians between any two points on the wave separated by 10.0 cm.
-
Lewis structures can be used to understand why some molecules react in certain ways. Write the Lewis structures for the reactants and products in the reactions de-scribed below. a. Nitrogen dioxide...
-
The most common type of exception to the octet rule are compounds or ions with central atoms having more than eight electrons around them. PF5, SF4, ClF3, and Br3- are examples of this type of...
-
SF6, ClF5, and XeF4 are three compounds whose central atoms do not follow the octet rule. Draw Lewis structures for these compounds.
-
Required information [The following information applies to the questions displayed below.] Dain's Diamond Bit Drilling purchased the following assets this year. Asset Drill bits (5-year) Drill bits...
-
Which of the following partnership items are not included in the self-employment income calculation? Ordinary income. Section 179 expense. Guaranteed payments. Gain on the sale of partnership...
-
Phantom Consulting Inc. is a small computer consulting business. The company is organized as a corporation and provides consulting services, computer system installations, and custom program...

Study smarter with the SolutionInn App


