In a study to see how closely gaseous ammonia obeys Boyles law, several volume measurements were made
Question:
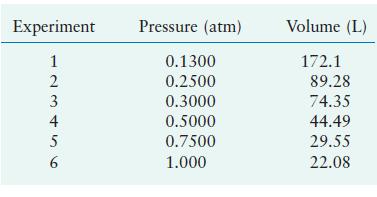
In a study to see how closely gaseous ammonia obeys Boyle’s law, several volume measurements were made at various pressures using 1.0 mol of NH3 gas at a temperature of 0°C. Using the results listed below, calculate the Boyle’s law constant for NH3 at the various pressures.
Transcribed Image Text:
Experiment 123456 2 4 5 Pressure (atm) 0.1300 0.2500 0.3000 0.5000 0.7500 1.000 Volume (L) 172.1 89.28 74.35 44.49 29.55 22.08
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
To determine how closely NH3 gas follows Boyles law under these conditions we calculate the ...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
a. Example 13-1: Batch Reactor with an Exothermic Reaction Wolfram 1. Adiabatic Case: Use Wolfram to see whether you can find a trajectory that is ready to ignite and whose trajectory looks like a...
-
In a study to examine the utility of using ammonia gas to sanitize animal feeds, researchers inoculated corn silage with a strain of Salmonella. Next, two petri dishes of 5 g of contaminated feed...
-
In a study to predict temperature from air pressure in a piston-cylinder device, 19 measurements were made of temperature in ¦f (y) and air pressure in psi (x). three models were fit: the...
-
In Pissarides's view of labor markets (job matching model), the focus is on finding a good ________. a. level of TFP (A) b. supply of money (M). c. match d. Wage
-
For the beam and loading shown, (a) Draw the shear and bending-moment diagrams, (b) Determine the maximum absolute values of the shear and bending moment. 150 lb 100 I 100 Ib 12 lvin. 12 lbvin. tra...
-
Suppose a lady gives her 10-year old son $100 on Christmas. She asks him to share this amount with his younger sister. He can offer her any amount between $0 and $100 but if she refuses to accept...
-
How do you transform a normal random variable to a standard normal random variable? LO8
-
If Webster had made the chowder herself from a recipe that she had found on the Internet, could she have successfully brought an action against its author for a breach of the implied warranty of...
-
Computers Inc. sells personal computers as well as home Internet service. On July 1, 2019, Computers sold a computer with a two-year Internet connection service contract. The customer paid $1,950...
-
A sample of hydrogen gas (H 2 ) has a volume of 8.56 L at a temperature of 0C and a pressure of 1.5 atm. Calculate the moles of H 2 present in this gas sample.
-
* The formate ion, \(\mathrm{CHO}_{2}{ }^{-}\), forms ionic compounds with many metal ions. Assume that \(9.7416 \mathrm{~g} \mathrm{M}\left(\mathrm{CHO}_{2}ight)_{2}\) (where \(\mathrm{M}\)...
-
Use a calculator to find the value of expression rounded to two decimal places. csc -1 (-3)
-
Everyone at some point has had issues with time management and procrastination in their work life, academic life and social life. How have you been handling time management issues in your life? Have...
-
You want to make three peanut butter and jelly sandwiches. What is the best way to make them that's consistent with an agile mindset? Create a sandwich assembly line, applying all the peanut butter...
-
1 pts Joan Reed exchanges commercial real estate that she owns for other commercial real estate, plus $50,000 cash. The following additional information pertains to this transaction: Property given...
-
It is believed that 86% of Padres fans would have liked Trevor Hoffman to remain in San Diego to finish out his career as a San Diego Padre. You would like to simulate asking 10 Padres fans their...
-
The videos below cover why American higher education, including public colleges and universities, is so expensive. They also explore factors that have resulted in the current student loan debt...
-
When you give a casino $5 for a bet on the number 7 in roulette, you have a 1/38 probability of winning $175 and a 37/38 probability of losing $5. What is your expected value? In the long run, how...
-
SCHEDULE OF COST OF GOODS MANUFACTURED The following information is supplied for Sanchez Welding and Manufacturing Company. Prepare a schedule of cost of goods manufactured for the year ended...
-
Repeat Example 15.24, except for a feed containing 400 ppm (by weight) of CaCl2 and 50 ppm of NaCl. Example 15.24 Hard water containing 500 ppm (by weight) MgCO 3 and 50 ppm NaCl is to be softened at...
-
In Examples 15.13 and 15.18, benzene is adsorbed from air at 70 F in a 6-ft-high bed of silica gel and then stripped with air at 145F. If the bed height is changed to 30 ft, the following data are...
-
A 55 mol% propane and 45 mol% propylene gas mixture is to be separated into products containing 10 and 90 mol% propane by adsorption in a continuous, countercurrent system operating at 25C and 1 atm....
-
Cash from Operating Activities: ______________ Cash from Investing Activities: ______________ Cash from Financing Activities: ______________ Problem 2: Financial Ratios The GAP Macys 1 Current Ratio...
-
On January 1, 2021, Winky Enterprises issued 12% bonds dated January 1, 2021, with a face amount of $2,800,000. The bonds mature in 2030 (10 years). For bonds of similar risk and maturity, the market...
-
Using the following accounts and balances, prepare the stockholders' equity vection of the balance sheet. Pilty thousand shares of common stock are authorised, and 1,000 shares have been recoured,...

Study smarter with the SolutionInn App


