In the presence of light, chlorine can substitute for one (or more) of the hydrogens in an
Question:
In the presence of light, chlorine can substitute for one (or more) of the hydrogens in an alkane. For the following reactions, draw the possible monochlorination products.
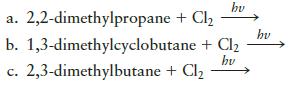
Transcribed Image Text:
hv a. 2,2-dimethylpropane + Cl₂ b. 1,3-dimethylcyclobutane + Cl₂ hv 2,3-dimethylbutane + Cl₂ C. hv
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 77% (9 reviews)
In the presence of light chlorine can substitute for one or more of the ...View the full answer

Answered By

BillClinton Muguai
I have been a tutor for the past 5 years. I have experience working with students in a variety of subject areas, including computer science, math, science, English, and history. I have also worked with students of all ages, from elementary school to college. In addition to my tutoring experience, I have a degree in education from a top university. This has given me a strong foundation in child development and learning theories, which I use to inform my tutoring practices.
I am patient and adaptable, and I work to create a positive and supportive learning environment for my students. I believe that all students have the ability to succeed, and it is my job to help them find and develop their strengths. I am confident in my ability to tutor students and help them achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In the presence of light, chlorine can substitute for one (or more) of the hydrogens in an alkane. For the following reactions, draw the possible monochlorination products. hr 2,2-dimethylpropane Cl2...
-
In Data A.1 on page 136, we introduce a study in which mice are randomly separated into one group on a normal light dark cycle and one group with bright light on all the time. Although the mice ate...
-
In our development of consumer theory, we made a big point about the fact that neoclassical economics does not put much stock in the idea of cardinally measuring utility (in terms of units of...
-
Three disease-carrying organisms decay exponentially in lake water according to the following model: Estimate the initial population of each organism (A, B, and C) given the followingmeasurements:...
-
In Exercises 20, (a) Find the five-number summary, and (b) Draw a box-and-whisker plot that represents the data set. 2 7 1 3 1 2 8 9 9 2 5 4 7 3 7 5 4 7 2 3 5 9 5 6 3 9 3 4 9 8 8 2 3 9 5
-
What properties can you set for a text string in a Text?
-
Are employees in the information systems-information technology area well qualified?
-
Ashes Divide Corporation has bonds on the market with 14.5 years to maturity, a YTM of 6.8 percent, and a current price of $924. The bonds make semiannual payments. The coupon rate on these bonds...
-
If $7,000 was generated from operations, $1,000 was used for investing activities and $6,000 was generated from financing activities, the cash balance must have increased by: O A. $2,000. B. $12,000...
-
Golden Wedding Dress Company designs custom wedding dresses for brides to be. The person preparing the adjusting entries at year-end was unable to complete the adjustments due to illness. You have...
-
Alkenes and cycloalkanes are structural isomers of each other. Give an example of each using C 4 H 8 . Another common feature of alkenes and cycloalkanes is that both have restricted rotation about...
-
Cumene is the starting material for the industrial production of acetone and phenol. The structure of cumene is Give the systematic name for cumene. CH3 -CH CH3
-
Progressive Corporation acquired all of the outstanding stock of Static Company on June 30, 2013, by issuing 200,000 shares of its $1 par value common stock valued at $50 per share. Direct cash costs...
-
The State of Confusion Legislature passes the following statute: "The State Health Commissioner, when in their opinion, there is sufficient covid - 1 9 vaccine that has been approved by the Federal...
-
Case 1 Baum Co. has two processing departments: Fabrication and Assembly. In the Fabrication Department, metal is cut and formed into various components, which are then transferred to Assembly. The...
-
Your earlier Personal Leadership Assessment, you looked at two areas of your leadership experience, those who led you and those you led. You will again address these two items in your Personal...
-
What is required in this situation: Content slides explaining the qualitative and quantitative steps necessary in conducting a sensitivity analysis. How can a project's risk be incorporated into a...
-
What is "marketing"? What is the difference between "marketing" and the "marketing process"?is it different in your home country vs. North america? Q2. What is the difference between "demand",...
-
Solve each inequality. Give the answer using interval notation. +1 3
-
What are some of the possible sources of information about a company that could be used for determining the companys competitive stance?
-
Explain why water forms into beads on a waxed car finish.
-
Hydrogen peroxide (H2O2) is a syrupy liquid with a relatively low vapor pressure and a normal boiling point of 152.2oC. Rationalize the differences between these physical properties and those of...
-
List the major types of intermolecular forces in order of increasing strength. Is there some overlap? That is, can the strongest London dispersion forces be greater than some dipoledipole forces?...
-
Which of the following statements regarding traditional cost accounting systems is false? a. Products are often over or under cost in traditional cost accounting systems. b. Most traditional cost...
-
Bart is a college student. Since his plan is to get a job immediately after graduation, he determines that he will need about $250,000 in life insurance to provide for his future wife and children...
-
Reporting Financial Statement Effects of Bond Transactions (please show me how you got the answers) Lundholm, Inc., which reports financial statements each December 31, is authorized to issue...

Study smarter with the SolutionInn App


