Investigate whether the replacement of a carboncarbon double bond by single bonds is energetically favored by using
Question:
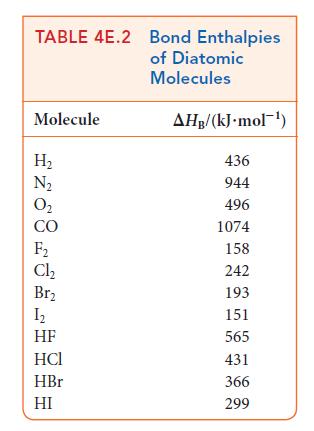
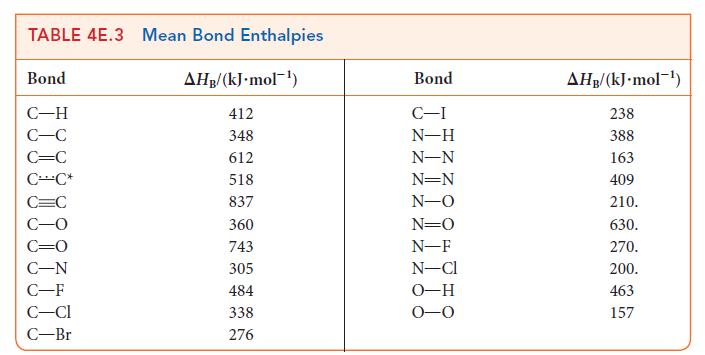
Investigate whether the replacement of a carbon–carbon double bond by single bonds is energetically favored by using Tables 4E.2 and 4E.3 to calculate the reaction enthalpy for the conversion of ethene, C2H4, to ethane, C2H6. The reaction is![]()
Transcribed Image Text:
H₂C=CH₂(g) + H₂(g) →CH3-CH3(g).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To calculate the reaction enthalpy for the conversion of ethene C2H4 to ethane C2H6 we need to consi...View the full answer

Answered By

Madhur Jain
I have 6 years of rich teaching experience in subjects like Mathematics, Accounting, and Entrance Exams preparation. With my experience, I am able to quickly adapt to the student's level of understanding and make the best use of his time.
I focus on teaching concepts along with the applications and what separates me is the connection I create with my students. I am well qualified for working on complex problems and reaching out to the solutions in minimal time. I was also awarded 'The Best Tutor Award' for 2 consecutive years in my previous job.
Hoping to get to work on some really interesting problems here.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The conversion of natural gas, which is mostly methane, into products that contain two or more carbon atoms, such as ethane (C2H6), is a very important industrial chemical process. In principle,...
-
The replacement of a planing machine is being considered by the Reardorn Furniture Company. (There is an indefinite future need for this type of machine.) The best challenger will cost $30,000 for...
-
Although ab initio HF calculations fail in predicting molecular atomization energies, one can still use HF energies to estimate energy changes for certain types of reactions. Recall that good...
-
Assume that you are purchasing an investment and have decided to invest in a company in the digital phone business. You have narrowed the choice to Best Digital, Corp., and Every Zone, Inc., and have...
-
A job order cost sheet for Rolen Company is shown below. Instructions(a) On the basis of the foregoing data answer the following questions.(1) What was the balance in Work in Process Inventory on...
-
Use the Table of Integrals on the Reference Pages to evaluate the integral. e2x - 1 dx
-
Record transactions involving cash dividends. (p. 449) AppendixLO1
-
1. Assuming that the distribution has not changed from what it was in the past year, what is the probability that the upload speed is a. Less than 1.0? b. Between 0.95 and 1.0? c. Between 1.0 and...
-
Required information The following information applies to the questions displayed below! Jessie Co. Issued $6 million face amount of 12.5%, 10-year bonds on April 1, 2019. The bonds pay interest on...
-
Suppose that you create two tiny systems consisting of three atoms each, and each atom can accept energy in quanta of the same magnitude. (a) How many distinguishable arrangements are there of two...
-
In an adiabatic process, no energy is transferred as heat. Indicate whether each of the following statements about an adiabatic process in a closed system is always true, always false, or true in...
-
You invest $50,000 in Germany when the exchange rate is $1.42/. Your investment gains 15 percent, and you subsequently exchange the euros back into dollars at a rate of $1.48/. What is your total...
-
Construct a 90% confidence interval for the population standard deviation o at Bank B. Bank B 4.2 5.4 5.9 6.1 6.6 7.7 7.7 8.6 9.3 10.0
-
Jamila Traders has a head office in Nanyuki and an autonomous branch in Thika. The trial balances of the head office and the branch as at 30 September 2014 were as follows: Head office Sh. Sh. Thika...
-
Poll Results in the Media USA Today provided results from a survey of 1144 Americans who were asked if they approve of Brett Kavanaugh as the choice for Supreme Court justice. 51% of the respondents...
-
ROI analysis using the DuPont model a. Firm A has a margin of 7%, sales of $980,000, and ROI of 19.6%. Calculate the firm's average total assets. b. Firm B has net income of $259,200, turnover of...
-
The test statistic of z = - 2.93 is obtained when testing the claim that p < 2/ 3. This is a left-tailed test. Using a 0.01 significance level, complete parts (a) and (b). a. Find the critical...
-
Compare phenotypes at the molecular, cellular, and organism levels for individuals who are homozygous for the hemoglobin allele, HbAHhA, and the sickle cell allele, HbSHbS.
-
Define a traverse in Surveying?
-
The heat capacity of a bomb calorimeter was determined by burning 6.79 g of methane (energy of combustion = 802 kJ/ mol CH4) in the bomb. The temperature changed by 10.8oC. a. What is the heat...
-
The combustion of 0.1584 g benzoic acid increases the temperature of a bomb calorimeter by 2.54oC. Calculate the heat capacity of this calorimeter. (The energy re-leased by combustion of benzoic acid...
-
Combustion of table sugar produces CO2(g) and H2O(l). When 1.46 g of table sugar is combusted in a constant-volume (bomb) calorimeter, 24.00 kJ of heat is liberated. a. Assuming that table sugar is...
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


