Name the following compounds: (a) CHCH (b) CHCH CHCHCH CHCH CHCHCH
Question:
Name the following compounds: 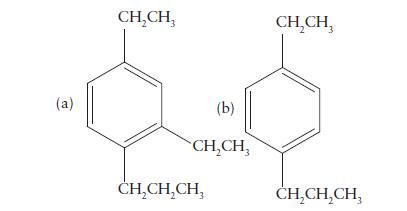
Transcribed Image Text:
(a) CHCH (b) CHCH CHCHCH CHCH CHCHCH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
The compounds shown are derivatives of benzene which is a hexagonal ring of carbon atoms with altern...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Name the following compounds by IUPAC rules: a. b. H-C CH,CH-CH
-
Name the following compounds by the IUPAC system: a. CH3CH=C(CH2CH2CH3)2 b. (CH3)2CHCH"CHCH3 c. g. CH3-C-C-CH-CH, h. k.
-
Name the following compounds and assign oxidation states to the halogens in them: (a) Fe(ClO3)3 (b) HClO2 (c) XeF6 (d) BrF5 (e) XeOF4 (f) HIO3.
-
The figure shows a coil with a sheet iron core with unlimited magnetic penetration and a rectangular cross-section. The coil is made of insulated copper wire with specific resistance p. Coil...
-
Design a parallel RLC circuit with R 1k that has the characteristic equation s 2 + 4 x 10 7 s + 4 x 10 14 = 0.
-
Lafayette Corporation, a client, requests that you compute the appropriate balance of its estimated liability for product warranty account for a statement as of June 30, 2011. Lafayette Corporation...
-
3. How would Honda R&Ds emphasis on recruiting recent graduates, rather than experienced automotive engineers, affect your job if you were the supervisor of these employees? Would you want Honda to...
-
1. Would you describe the exposure of the Sports Exports Company to exchange rate risk as transaction exposure? Economic exposure? Translation exposure? 2. Jim Logan is considering a change in the...
-
Under which of the following circumstances would it be most acceptable to use the Packback Period decision method (check all that apply) A. The project involves buying a piece of equipment that has a...
-
Sketch three repeating units of the polymer formed from (a) 1,1-dichloroethene; (b) 1-phenyl-2-methylethene; (c) CH 3 CH=CH 2 CF 3 .
-
Draw line structures of the following molecules and identify each as an alkane, alkene, or alkyne: (a) CH 3 CCCH 3 ; (b) CH 3 CH 2 CH 2 CH 3 ; (c) CH 2 CHCH 2 CH 3 ; (d) CH 3 CHCHCH 2 CCCH 3 ; (e) CH...
-
Company N, an accrual basis taxpayer, owes $90,000 to Creditor K. At the end of 2018, Company N accrued $7,740 interest payable on this debt. It didnt pay this liability until March 3, 2019. Both...
-
The balances of selected accounts of Casper Company on February 28, 20X1, were as follows: Sales $250,000 and Sales Returns and Allowances $4,000. The firm's net sales are subject to an 7 percent...
-
1. Draw and label force diagrams for the physics book and for the calculator. Add equality marks showing any equalities between force diagrams. Circle and label any Newton's third law pairs. (6 pts)...
-
Consider the Lincoln Tunnel, which was built in 1939 under the Hudson River in New York. Assume the tunnel to be empty with perfectly conducting walls and rectangular cross section with width 6.55 m...
-
Examine a well-known principal-agent contract, the sale of your home by a licensed realtor. You will use the following data to analyze this case. Your home is the typical home, approximately 1,875 sq...
-
i) Generate a third degree polynomial in x and y named g(x, y) that is based on your mobile number (Note: In case there is a 0 in one of the digits replace it by 3). Suppose your mobile number is...
-
How many grams of 50 wt% NaOH (FM 40.00) should be diluted to 1.00 L to make 0.10 M NaOH? (Answer with two digits.)
-
Assume you are the accountant for Catalina Industries. John Catalina, the owner of the company, is in a hurry to receive the financial statements for the year ended December 31, 20X1, and asks you...
-
The drive shaft AB of an automobile is to be designed as a thin-walled tube. The engine delivers 150 hp when the shaft is turning at 1000 rev>min. Determine the minimum thickness of the shafts wall...
-
The drive shaft AB of an automobile is made of a steel having an allowable shear stress of Ï allow = 8 ksi. If the outer diameter of the shaft is 2.5 in. and the engine delivers 200 hp to the...
-
When drilling a well at constant angular velocity, the bottom end of the drill pipe encounters a torsional resistance T A . Also, soil along the sides of the pipe creates a distributed frictional...
-
[ The following information applies to the questions displayed below ] Nauticat has two classes of stock authorized: $ 1 0 par preferred, and $ 1 par value common. As of the beginning of 2 0 2 1 , 1...
-
Selling is not the most important part of marketing. Explain why not
-
When direct materials are issued from the storeroom, are any entries made in the subsidiary records? Question 2 options: Increase raw material item record Decrease raw material item record No entry...

Study smarter with the SolutionInn App


