Nitroglycerin is a shock-sensitive liquid that detonates by the reaction Calculate the total volume of product gases
Question:
Nitroglycerin is a shock-sensitive liquid that detonates by the reaction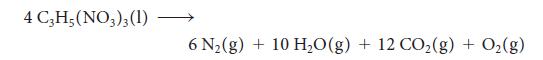
Calculate the total volume of product gases at 88.5 kPa and 175°C from the detonation of 1.00 lb (454 g) of nitroglycerin.
Transcribed Image Text:
4 C3H5(NO3)3 (1) 6 N₂(g) + 10 H₂O(g) + 12 CO₂(g) + O₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
First we need to calculate the moles of nitroglycerin The molar mass of nitroglycerin C3H5NO33 can b...View the full answer

Answered By

Jonas Araujo
I have recently received the degree of PhD. In Physics by the Universidade Federal do Maranhão after spending a term in Durham University, as I have been awarded a scholarship from a Brazilian mobility program. During my PhD. I have performed research mainly in Theoretical Physics and published works in distinguished Journals (check my ORCID: https://orcid.org/0000-0002-4324-1184).
During my BSc. I have been awarded a scholarship to study for a year in the University of Evansville, where I have worked in detection-analysis of photon correlations in the the Photonics Laboratory. There I was a tutor in Electromagnetism, Classical Mechanics and Calculus for most of that year (2012).
I am very dedicated, honest and a fast learner, but most of all, I value a job well done.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
In the following we use the assumption that the number of different atoms on earth is finite. Furthermore, we say that any possible composition of a finitely many atoms is called a molecule. Note...
-
On 1 January 20x1, INV Ltd acquired $1,000,000 of equity instrument for which it has elected to present changes in fair value in other comprehensive income. On the same day, INV Ltd purchased a put...
-
1-Which of the following items should be included when calculating portfolio performance? A) Income and contributions B) Capital gains/losses and distributions C) Income and distributions D) Capital...
-
Hines stored her furniture, including a grand piano, in Arnetts warehouse. Needing more space, Arnett stored Hiness piano in Butlers warehouse next door. As a result of a fire, which occurred without...
-
What is responsibility accounting? Explain the purpose of responsibility accounting.
-
The following table shows the ticket sales of the 15 highest grossing movies of all time in the United States, adjusted for inflation, in millions of dollars, as of May 5, 2019. Movie Ticket Sales...
-
Create a frequency distribution and graph for the ANES variable regarding removing a member of the Supreme Court. How could you use these results to argue that Americans are in favor of removing a...
-
An assembly station is asked to process 100 circuit boards per hour. It takes 20 minutes to receive the necessary components from the previous workstation. Completed circuit boards are placed in a...
-
wanson & Hiller, Inc., purchased a new machine on September 1 of the current year at a cost of $108,000. The machines estimated useful life at the time of the purchase was five years, and its...
-
Low-pressure gauges in research laboratories are occasionally calibrated in inches of water (inH 2 O). Given that the density of mercury at 15C is 13.6 g cm 3 and the density of water at that...
-
The Haber process for the synthesis of ammonia is one of the most significant industrial processes for the well-being of humanity. It is used extensively in the production of fertilizers as well as...
-
In Exercises test for convergence or divergence and identify the test used. n=0 5 718 L n
-
Activity 1.4: When Less Becomes More For this activity, refer to the images shown. This is an activity which was performed for you if you do not have available two identical mirrors at home. But if...
-
! Required information [The following information applies to the questions displayed below.] Aces Incorporated, a manufacturer of tennis rackets, began operations this year. The company produced...
-
During the early part of winter, one morning, two hunters decided to go quail hunting on a property where the owner had given them permission to hunt. A nearby forest ranger saw the hunters and...
-
Required information [The following information applies to the questions displayed below.] Trini Company set the following standard costs per unit for its single product. Direct materials (30 pounds...
-
A horticulturist knows that the weights of honeybees that have previously visited her orchard are normally distributed with a mean of 0.87 grams, and a population standard deviation of 0.15 grams....
-
A cross is made between a pea plant that has constricted pods (a recessive trait; smooth is dominant) and is heterozygous for seed color (yellow is dominant to green) and a plant that is heterozygous...
-
Suppose that A is an m n matrix with linearly independent columns and the linear system LS(A, b) is consistent. Show that this system has a unique solution.
-
What acid and what base would react in aqueous solution so that the following salts appear as products in the formula equation? Write the balanced formula equation for each reaction. a. Potassium...
-
The drawings below represent aqueous solutions. Solution A is 2.00 L of a 2.00-M aqueous solution of copper(II) nitrate. Solution B is 2.00 L of a 3.00-M aqueous solution of potassium hydroxide. a....
-
What mass of Na 2 CrO 4 is required to precipitate all of the silver ions from 75.0 mL of a 0.100-M solution of AgNO 3 ?
-
When preparing government-wide financial statements, the modified accrual based governments funds are adjusted. Please show the adjustments (in journal entry form with debits and credits) that would...
-
I need help finding the callable price and call value
-
On 31 October 2022, the owner took goods for his son as a birthday gift. The cost price of the goods was R15 000

Study smarter with the SolutionInn App


