Question:
Osmometry has been widely used in the polymer industry because it is a sensitive technique for determining the huge molar masses of polymer molecules. Now imagine that you are a polymer chemist; you have devised a new way to make polyethylene and wish to know the molar mass of your new material. The osmotic pressure due to 2.20 g of polyethylene (PE) dissolved in enough benzene to produce 100.0 mL of solution was 1.10 * 10–2 atm at 25°C. Calculate the molar mass of the polymer, which is a nonelectrolyte. The answer will be an average molar mass as not all the polymer molecules are the same length.
ANTICIPATE Because so many atoms are linked together in each polymer molecule, you should expect a high molar mass.
PLAN Use the procedure for osmometry in Toolbox 5E.1. Because polyethylene is a nonelectrolyte, i = 1. Use R in the units that match the data—in this case, liters and atmospheres.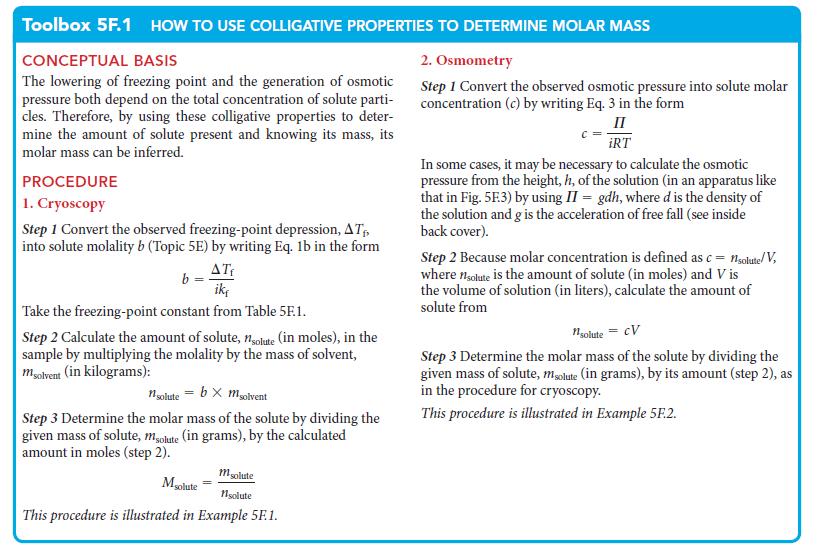
Transcribed Image Text:
Toolbox 5F.1 HOW TO USE COLLIGATIVE PROPERTIES TO DETERMINE MOLAR MASS
CONCEPTUAL BASIS
The lowering of freezing point and the generation of osmotic
pressure both depend on the total concentration of solute parti-
cles. Therefore, by using these colligative properties to deter-
mine the amount of solute present and knowing its mass, its
molar mass can be inferred.
PROCEDURE
1. Cryoscopy
Step 1 Convert the observed freezing-point depression, AT₁,
into solute molality b (Topic 5E) by writing Eq. 1b in the form
ATF
ik,
b
Take the freezing-point constant from Table 5F.1.
Step 2 Calculate the amount of solute, nsolute (in moles), in the
sample by multiplying the molality by the mass of solvent,
msolvent (in kilograms):
nsolute = bx msolvent
Step 3 Determine the molar mass of the solute by dividing the
given mass of solute, meolute (in grams), by the calculated
amount in moles (step 2).
msolute
Msolute
1solute
This procedure is illustrated in Example 5F.1.
2. Osmometry
Step 1 Convert the observed osmotic pressure into solute molar
concentration (c) by writing Eq. 3 in the form
II
iRT
In some cases, it may be necessary to calculate the osmotic
pressure from the height, h, of the solution (in an apparatus like
that in Fig. 5F.3) by using II = gdh, where d is the density of
the solution and g is the acceleration of free fall (see inside
back cover).
Step 2 Because molar concentration is defined as c = 1solute/V,
where nolute is the amount of solute (in moles) and V is
the volume of solution (in liters), calculate the amount of
solute from
1solute = CV
Step 3 Determine the molar mass of the solute by dividing the
given mass of solute, msolute (in grams), by its amount (step 2), as
in the procedure for cryoscopy.
This procedure is illustrated in Example 5F.2.







