You have made up a solution of known molarity but now realize that you need to know
Question:
You have made up a solution of known molarity but now realize that you need to know the molality instead. Find the molality of sucrose, C12H22O11, in 1.06 m C12H22O11(aq), which is known to have density 1.140 g · mL–1.
ANTICIPATE The mass of 1 L of aqueous solution is close to 1 kg, so the numerical value of the molality can be expected to be close to that of the molarity, but with different units, of course.
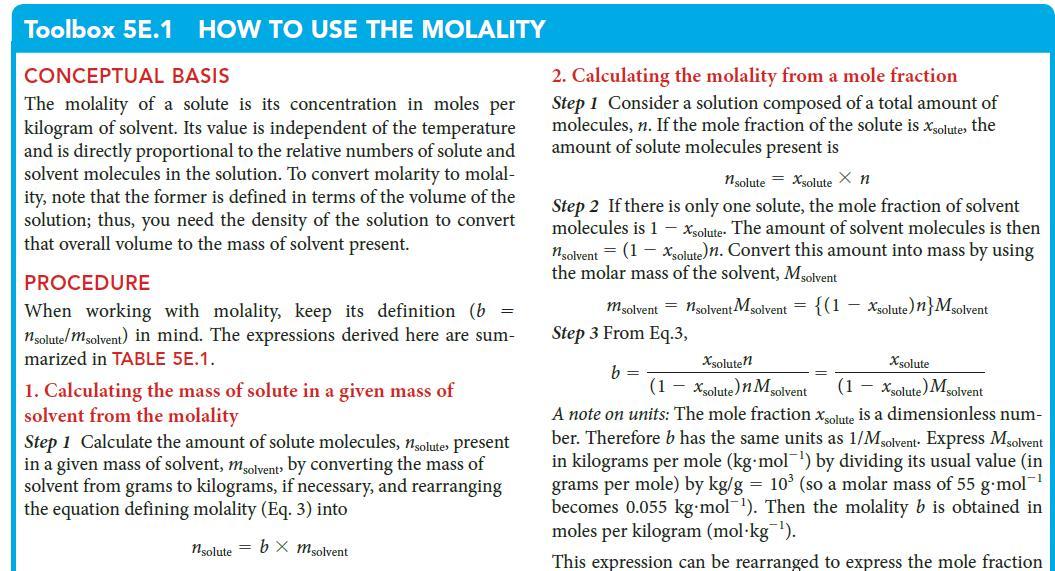
PLAN Use procedure 3 in Toolbox 5E.1, taking note of the discussion of units.
Transcribed Image Text:
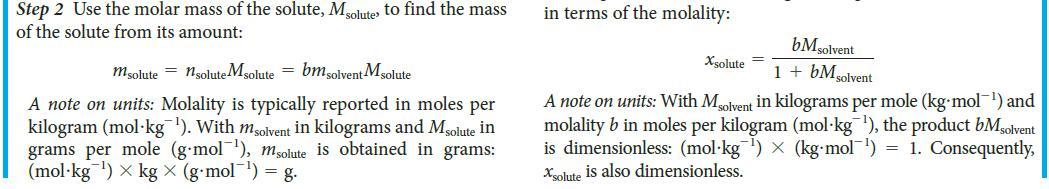
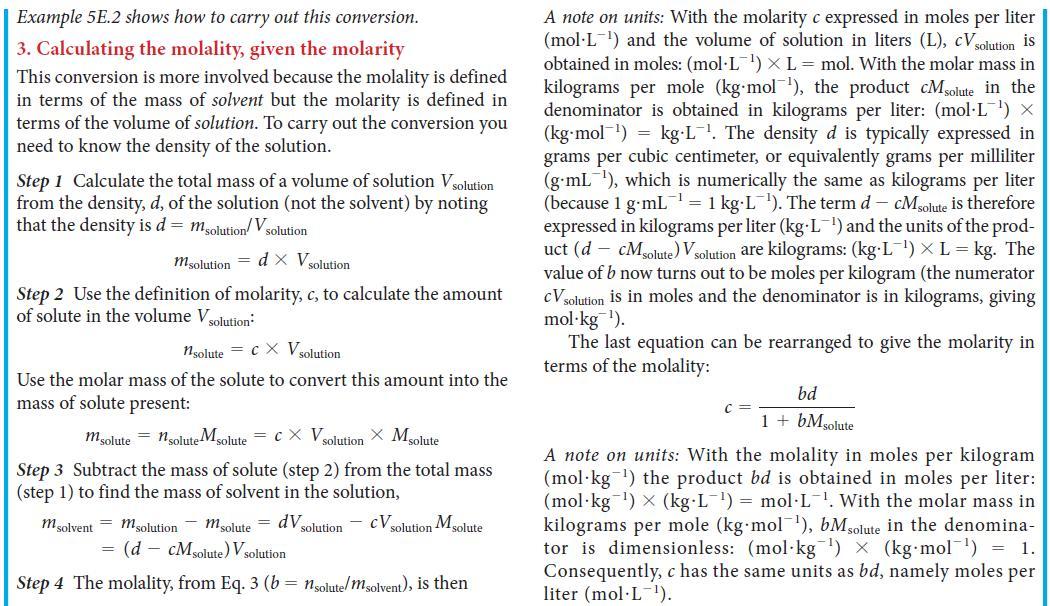
Toolbox 5E.1 HOW TO USE THE MOLALITY CONCEPTUAL BASIS The molality of a solute is its concentration in moles per kilogram of solvent. Its value is independent of the temperature and is directly proportional to the relative numbers of solute and solvent molecules in the solution. To convert molarity to molal- ity, note that the former is defined in terms of the volume of the solution; thus, you need the density of the solution to convert that overall volume to the mass of solvent present. PROCEDURE When working with molality, keep its definition (b = nsolute/msolvent) in mind. The expressions derived here are sum- marized in TABLE 5E.1. 1. Calculating the mass of solute in a given mass of solvent from the molality Step 1 Calculate the amount of solute molecules, nsolute present in a given mass of solvent, msolvent by converting the mass of solvent from grams to kilograms, if necessary, and rearranging the equation defining molality (Eq. 3) into nsolute = bx msolvent 2. Calculating the molality from a mole fraction Step 1 Consider a solution composed of a total amount of molecules, n. If the mole fraction of the solute is xsolute, the amount of solute molecules present is 1solute = Xsolute X n Step 2 If there is only one solute, the mole fraction of solvent molecules is 1 - Xsolute. The amount of solvent molecules is then nsolvent = (1 - Xsolute)n. Convert this amount into mass by using the molar mass of the solvent, Msolvent m solvent = nsolvent Msolvent = {(1 Step 3 From Eq.3, b = - Xsolute) n} Msolvent Xsolute Xsoluten (1-xsolute)n M solvent (1 - Xsolute) Msolvent A note on units: The mole fraction solute is a dimensionless num- ber. Therefore b has the same units as 1/Msolvent Express Msolvent in kilograms per mole (kg mol¹) by div its usual value (in grams per mole) by kg/g = 10³ (so a molar mass of 55 g-mol™¹ becomes 0.055 kg-mol¹). Then the molality b is obtained in moles per kilogram (mol kg ¹). This expression can be rearranged to express the mole fraction
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Note that a molar mass of 3423 gmol is equivalent to 03423 kgmol ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
A colleague has been doing an experiment in which it was important to know the mole fractions of the components of a solution. You want to use the same solution, but you have in mind an experiment in...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
MiSTi, like many small technology companies, was born as an extension of the founder's special technical skills in the highly specialized field of "micro-switch" technology in the late 90's. Under...
-
State whether or not each of the following events would result in a liability being recognised in the accounts at 30 June. 1. Taxes for the year ended 30 June, which are not payable until October. 2....
-
You are asked to construct a parallel-plate, air-gap capacitor that will store 100 kJ of energy. (a) What minimum volume is required between the plates of the capacitor? (b) Suppose you have...
-
Find expressions for the maximum values of the spring force and deflection y of the impact system shown in the figure. Can you think of a realistic application for this model? WWH
-
What are the major sources of marketing information? What information do you think is vital at the unit level? At the headquarters level?
-
Managers are often concerned about the impact on reported profits of any actions recommended by the tax planning department. Explain why.
-
Peng Company is considering buying a machine that will yield income of $2,300 and net cash flow of $16,200 per year for three years. The machine costs $48,900 and has an estimated $7,200 salvage...
-
On January 1, 2021, the general ledger of Grand Finale Fireworks includes the following account balances: During January 2021, the following transactions occur: January 2 Issue an additional 2,000...
-
The equilibrium constant for the reaction 2 NO(g) + O 2 (g) 2 NO 2 (g) is K = 2.5 * 10 10 at 500. K. Find the value of K for each of the following reactions at the same temperature. = NO(g) + O(g)...
-
Osmometry has been widely used in the polymer industry because it is a sensitive technique for determining the huge molar masses of polymer molecules. Now imagine that you are a polymer chemist; you...
-
For the input stage of the 741 op-amp, assume B-E breakdown voltages of \(5 \mathrm{~V}\) for the npn devices and \(50 \mathrm{~V}\) for the pnp devices. Estimate the differential input voltage at...
-
Find the explained variation for the paired data. The equation of the regression line for the paired data below is y = 5.18286 + 3.33937x. X 972 23 34 4 22 17 y 43 35 16 21 23 102 81
-
5. The vertical stress at a point is 28 kPa, while the horizontal stress is 14 kPa. shear stress on the horizontal plane is +4 kPa. The (a) Draw the Mohr's circle of stress and show the pole point...
-
I need assistance with the below questions for my HIM 5370 at texas State University Case Mix Table: 4. Complete the Case Mix table shown below. Calculate the case mix for each month. The table below...
-
The probability that a printing press will print a book with no errors is 78%. The company is about to process an order of 30 books. Round decimals to 3 places or percentages to 1 decimal place. 9....
-
alculate Product Costs, using JOB COSTING SYSTEM. Please SHOW CALCULATION. Dream Chocolate Company: Choosing a Costing System TABLE 1 Typical Prices and Costs of Chocolate 641 1.25 oz. Bar 3.0 oz....
-
As data loss admissions become more widespread, how would they affect consumers' willingness to share information with corporations?
-
PC Contractors, Inc., was an excavating business in Kansas City, Missouri. Union Bank made loans to PC, subject to a perfected security interest in its equipment and other assets, including...
-
Propose a stepwise mechanism for the following transformation. Et Me 1) Excess EtMgBr Me OH 2) H,0 'Et
-
Predict the products for each of the following: a. b. c. d. 1) Hg(OAc), 2) NABH, 1) Hg(OAc), e 2) NaBH,
-
Starting with benzene and using any other necessary reagents of your choice, design a synthesis for each of the following compounds. Each compound has a Br and one other substituent that we did not...
-
explain in excel please For a particular product the price per unit is $6. Calculate Revenue if sales in current period is 200 units. Conduct a data analysis, on revenue by changing the number of...
-
Hall Company sells merchandise with a one-year warranty. In the current year, sales consist of 35,000 units. It is estimated that warranty repairs will average $10 per unit sold and 30% of the...
-
Q 4- Crane Corporation, an amusement park, is considering a capital investment in a new exhibit. The exhibit would cost $ 167,270 and have an estimated useful life of 7 years. It can be sold for $...

Study smarter with the SolutionInn App


