The equilibrium constant for the reaction 2 NO(g) + O 2 (g) 2 NO 2 (g)
Question:
The equilibrium constant for the reaction 2 NO(g) + O2(g) ⇌ 2 NO2(g) is K = 2.5 * 1010 at 500. K. Find the value of K for each of the following reactions at the same temperature.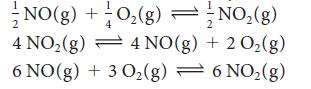
Transcribed Image Text:
= NO(g) + O₂(g) NO₂(g) 4 NO(g) + 2 O₂(g) 4 NO₂(g) 6 NO(g) + 3 0₂(g) = 6 NO₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Given the reaction 2NOg O2g 2NO2g with equilibrium constant K 25 1010 at 500 K we can find t...View the full answer

Answered By

Rehab Rahim
I am well versed in communicating and teaching in areas of all business subjects. I have helped many students in different ways from answering answers to writing their academic papers.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
You have been assigned the task of measuring the equilibrium constant for the reaction N 2 O 4 2NO 2 as a function of temperature. To do so, you evacuate a rigid 2-liter vessel equipped with a...
-
The equilibrium constant for the reaction H2 + at 1 atm and 1500C is given to be K. Of the reactions given below, all at 1500C, the reaction that has a different equilibrium constant is (a) H2 + 12O2...
-
Find the equilibrium constant for the reaction 2NO + O2 2NO2 from the elementary reactions in Table A.10 to answer which of the nitrogen oxides, NO or NO2; is the more stable at...
-
In Exercises 2748, find the open intervals where the functions are concave upward or concave downward. Find any inflection points. f(x) = 2e x2
-
Consider two parallel-plate capacitors, C1 and C2, that are connected in parallel. The capacitors are identical except that C2 has a dielectric inserted between its plates. A voltage source of 200 V...
-
For the curved beam shown, F = 30 kN. The material is steel with E = 207 GPa and G = 79 GPa. Determine the relative deflection of the applied forces. 40 50 10 Section A-A (All dimensions in...
-
How is marketing intelligence gathered?
-
Collins Corporation issued 10,000 shares of no-par common stock for $20 per share. Collins also issued 2,000 shares of $50 par, 5 percent noncumulative preferred stock at $55 per share. Required...
-
Assignment Information | The trial balance of Pacific Security Services, Incorporated as of January 1 , Year 3 , had the following normal balances: Pacific Security Services Trial Balance Account...
-
1. Based on prior experience, GBI estimates that approximately % of the net credit sales (gross credit sales minus returns of credit sales) for the month will become bad debt. GBI writes off bad...
-
Which do you expect to have the higher vapor pressure at room temperature, octane, C 8 H 18 , or butane, C 4 H 10 ? Why?
-
You have made up a solution of known molarity but now realize that you need to know the molality instead. Find the molality of sucrose, C 12 H 22 O 11 , in 1.06 m C 12 H 22 O 11 (aq), which is known...
-
Targaryen Corporation has a target capital structure of 70 percent common stock, 5 percent preferred stock, and 25 percent debt. Its cost of equity is 10 percent, the cost of preferred stock is 5...
-
Canis Major Veterinary Supplies Inc. DuPont Analysis Ratios Value Correct/Incorrect Ratios Value Correct/Incorrect Profitability ratios Gross profit margin (%) 50.00 Correct Asset management ratio...
-
The entrance to Salt Lake City (elevation 3,075 ft at the point of crossing) is grade separated from interstate highway (elevation 3,050 ft at the point of crossing) and will have to be connected....
-
McKnight Handcraft is a manufacturer of picture frames for large retailers. Every picture frame passes through two departments: the assembly department and the finishing department. This problem...
-
A deep reinforced concrete member carries two members with factored loads as shown in Figure 2. Material properties: fy 400 MPa, f'e = 50 MPa. a) Sketch a feasible strut and tie model indicating the...
-
If triangles ABC and DFG are similar triangles and side DF = 218, what is the value of side DG?
-
How should a corporation decide the appropriate level of resources to devote to securing its data?
-
United Business Forms capital structure is as follows: Debt ............................................ 35% Preferred stock ........................... 15 Common equity .......................... 50...
-
Propose a plausible mechanism for each of the following transformations. a. b. c. d. e. f. OH 1) EtMgBr 2) H20
-
What product do you expect when tetrahydrofuran is heated in the presence of excess HBr?
-
Compound B has molecular formula C 6 H 10 O and does not possess any bonds. When treated with concentrated HBr, cis-1, 4-dibromocyclohexane is produced. Identify the structure of compound B.
-
Production numbers for 2 shifts are shown. The shift supervisor of Shift 2 insists to the production manager that her operators are more productive than the ones on Shift 1. Using a confidence level...
-
In a class, the scores that students got are as shown. What are the 25, 50, 75 and 100th percentiles for the data? 84 84 98 80 89 83 85 56 85 84 84 74 84 81 83 80 45 86 67 79 81 78 76 85 83 77 86 83...
-
Number of points made by Teams A and B are shown. Which statement is true based on running the F-Test Two-Sample for Variances in the Data Analysis pack in Excel? Use a confidence level of 10% to...

Study smarter with the SolutionInn App


