Phosphorus pentachloride, PCl 5 , is used to convert alcohols (such as CH 3 CH 2 OH)
Question:
Phosphorus pentachloride, PCl5, is used to convert alcohols (such as CH3CH2OH) to alkyl chlorides (such as CH3CH2Cl). If you were an industrial chemist, you might be asked to prepare some PCl5 by the reaction of PCl3 and Cl2. At elevated temperatures, however, PCl5 decomposes to these starting materials, so it will be important to estimate the equilibrium composition of the reaction mixture. Suppose that you place 3.12 g of PCl5 in a reaction vessel of volume 500. mL and allow the sample to reach equilibrium with its decomposition products PCl3 and Cl2 at 250°C, when K = 78.3 for the reaction PCl5 (g) ⇌ PCl3 (g) + Cl2(g). Find the composition of the equilibrium mixture.
ANTICIPATE Because the equilibrium constant is neither very small nor very large, you should expect that the partial pressures of reactants and products at equilibrium will be similar to each other.
PLAN Use the general procedure set out in Toolbox 5I.1. Because data are in liters and pressures are expected in bar, use the value of R that matches these units (namely, L · bar · K–1 · mol–1).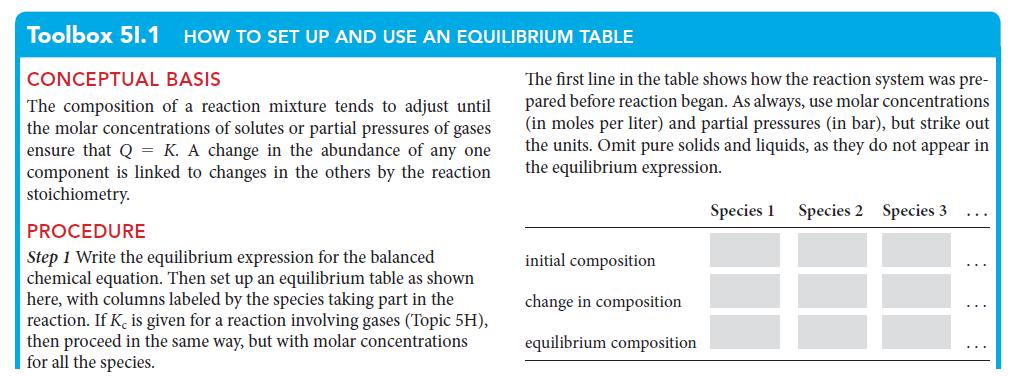
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





