Polystyrene can be made more rigid by copolymerizing styrene with divinylbenzene, What purpose does the divinylbenzene serve?
Question:
Polystyrene can be made more rigid by copolymerizing styrene with divinylbenzene,
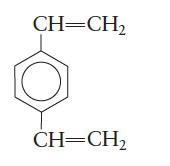
What purpose does the divinylbenzene serve? Why is the copolymer more rigid?
Transcribed Image Text:
CH=CH₂ CH=CH₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
What purpose does the divinylbenzene serve The divinylbenzene serves as a crossl...View the full answer

Answered By

Abigael martinez
I have been a tutor for over 3 years and have had the opportunity to work with students of all ages and backgrounds. I have a strong belief that all students have the ability to learn and succeed if given the right tools and support. I am patient and adaptable, and I take the time to get to know each student's individual learning style in order to best support their needs. I am confident in my ability to help students improve their grades and reach their academic goals.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Polystyrene can be made more rigid by copolymerizing styrene with divinylbenzene, What purpose does the divinylbenzene serve? Why is the copolymer more rigid? CH=CH2 CH=CH,
-
What purpose does the Designated Order Turnaround system (SuperDOT) serve on the New York Stock Exchange?
-
The County of Everstone is a large county in a densely populated state in the United States. This county has a total of 34 incorporated cities within its boundaries. Overall, the county has a...
-
Jellio, Maker of Whimsical Furnishings, Considers Growth. Start by asking yourself, who is the customer for this product, not who is currently buying it. There is a difference. Then, where would they...
-
In Exercises 40, the midpoints A, B, and C are marked on the histogram. Match them with the indicated z-scores. Which z-scores, if any, would be considered unusual? z = 0.77 z = 1.54 z = -1.54...
-
What is a window?
-
Is information updated regularly?LO1
-
The following circuit operates if and only if there is a path of functional devices from left to right. The probability that each device functions is as shown. Assume that the probability that a...
-
perpetual inventory using FIFO Perpetual Inventory Using FIFO Beginning inventory, purchases, and sales data for portable game players are as follows: Apr. 1 Inventory 80 units @ $96 10 Sale 54 units...
-
1. Mr. Albert has a piece of property he has used in his business for 25 years. He is moving his business across the country and needs to get rid of the real estate he currently owns, as he won't be...
-
Aqueous solutions of amino acids are buffered solutions. Why?
-
Super glue contains methyl cyanoacrylate which readily polymerizes on exposure to traces of water or alcohols on the surfaces to be bonded together. The polymer provides a strong bond between the two...
-
A machine is purchased January 1 at a cost of $77,000. It is expected to serve for eight years and have a salvage value of $5,000. REQUIRED Prepare a schedule showing depreciation for each year and...
-
As a project manager it is important to utilize the right tool at the right time. When it comes to managing quality on projects, this is no exception. Identify three 'Total Quality Tools' that you...
-
Describe 2 change models that you could use to create change in an organization. Choose 1 of the models that you think would be most successful in an organization, and analyze reasons why you chose...
-
During the current year, Rothchild, Inc., purchased two assets that are described as follows. Heavy Equipment Purchase price, $375,000. Expected to be used for 10 years, with a residual value at the...
-
Regarding the Mozilla case, assume that Communities of Practice start to arise spontaneously around topics that are related to the visualizations in the Portal at Mozilla. What do you think is the...
-
Regarding Issues That Affect Recruitment, how would you proceed as the assistant superintendent for human resources in a school district that is experiencing a shortage of qualified applicants for...
-
Solve each inequality. Give the solution set in interval notation. |3x - 4| < 2
-
We all experience emotions, but some people disguise their true feelings better than others. Do you think this is a helpful or harmful thing to do? Under what conditions do you think it would be most...
-
Dry nitrogen gas is bubbled through liquid benzene (C6H6) at 20.0oC. From 100.0 L of the gaseous mixture of nitrogen and benzene, 24.7 g of benzene is condensed by passing the mixture through a trap...
-
A sample of dry nitrogen gas weighing 100.0 g is bubbled through liquid water at 25.0oC. The gaseous mixture of nitrogen and water vapor escapes at a total pressure of 700. torr. What mass of water...
-
The molar enthalpy of vaporization of water at 373 K is 41.16 kJ/mol. What fraction of this energy is used to change the internal energy of the water, and what fraction is used to do work against the...
-
September 1 . Purchased a new truck for $ 8 3 , 0 0 0 , paying cash. September 4 . Sold the truck purchased January 9 , Year 2 , for $ 5 3 , 6 0 0 . ( Record depreciation to date for Year 3 for the...
-
Find the NPV for the following project if the firm's WACC is 8%. Make sure to include the negative in your answer if you calculate a negative. it DOES matter for NPV answers
-
What is the value of a 10-year, $1,000 par value bond with a 12% annual coupon if its required return is 11%?

Study smarter with the SolutionInn App


