Predict the products of each of the following reactions and then balance each equation: (a) Mg (OH)
Question:
Predict the products of each of the following reactions and then balance each equation: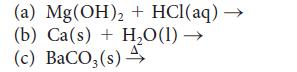
Transcribed Image Text:
(a) Mg (OH)₂ + HCl(aq) — (b) Ca(s) + H₂O(1)→ (c) BaCO3(s) 4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
a MgOHs 2 HClaq ...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Predict the products of each of the following reactions and then balance each equation: (a) Mg(s) + Br(1) (b) BaO(s) + Al(s) (c) CaO(s) + SiO (s)
-
Predict the products of each of the following reactions. (a) (b) (c) (d) OH Cl pyridine OH (1) NaH (2) CH2l HBr OH HNOg, H2SO4 H3C
-
Predict the major products of each of the following reactions and then balance the equations: (a) FeCrO4(s) + C(s) A (b) CrO (s) + HO*(aq) (c) MnO (s) + Al(s) A
-
Types of audit procedures Audit procedures are used to gatherevidence to support the auditors conclusions on the fairpresentation of a companys financial statements. Procedures can beperformed...
-
Look again at Table 4.7. a. How do free cash flow and present value change if asset growth rate is only 15 percent in years 1 to 5? If value declines, explain why. b. Suppose the business is a...
-
Determine the coefficients {h(n)} of a high pass linear phase FIR filter of length M = 4 which has an anti symmetric unit sample response h(n) = -h(M ? 1 ? n) and a frequency response that satisfies...
-
Figures 5.36 and 5.39 schematically show the operation or function of heat engines and heat movers, respectively. An alternative way to capture the information and meaning contained in these...
-
Park Manufacturing Company has been producing three products: frisbees, volleyballs, and croquet. Now that the plant has been shifted to an assembly-line operation, a fourth product, bocce, has been...
-
Kimmel, Accountini 7e PRINCIPLES OF ACCOUNTING (ACCT 2101 / 210 Anime Gradebook ORION Downloadable textbook CALATOR FLEST INTERVIESTO NEET Bale Exercise 25-01 is correct. Try agan Commiscering...
-
Suggest a Lewis structure for B 4 H 10 and deduce the formal charges on the atoms. There are four BHB bridges.
-
Lead azide, Pb(N 3 ) 2 , is used as a detonator. (a) What volume of nitrogen at STP (1 atm, 0 C) does 1.5 g of lead azide produce when it decomposes into lead metal and nitrogen gas? (b) Would 1.5 g...
-
What is a bailment? Describe three bailments in which you have been involved.
-
Moving Inc. wants to develop an activity flexible budget for the activity of moving materials. Moving Inc. uses forklifts to move materials from receiving to storeroom and then to production. The...
-
We are in the tail end of Quarter 3 earnings reporting season in the U.S. markets. Roughly 60 percent of companies that have reported their Q3 earnings so far have reported negative earnings relative...
-
Below is a running shock tube illustration. 0.1 0.0 | 0.0 4 4 Diaphragm 1 0.5 Image: Shock tube Initial setup 1 3 2 1 Expansion Head Expansion Tail Slip Shock Surface Image: Running Shock Tube...
-
As you may remember, Holiday Tree Services, Inc. (HTS) has recently entered into a contract with Delish Burger (Delish), whereby HTS is to supply and decorate a Christmas tree in each of Delish...
-
Understanding various types of leadership styles is important in order to determine personal leadership styles. Reflection: Answer both Compare and contrast 2 leadership styles. State the...
-
Use CRIME4.RAW for this exercise. (i) Reestimate the unobserved effects model for crime in Example 13.9 but use fixed effects rather than differencing. Are there any notable sign or magnitude changes...
-
Use of the contraceptive Depo Provera appears to triple women's risk of infection with chlamydia and gonorrhea , a study reports today. An estimated 20 million to 30 million women worldwide use Depo...
-
Using ζ as a variational parameter in the normalized function allows one to vary the size of the orbital. Show this by calculating the probability of finding the electron inside a sphere...
-
The overlap integral for Ï g and Ï u as defined in Section 23.3 is given by Plot S ab as a function of R/a 0 for ζ = 0.8, 1.0, and 1.2. Estimate the value of R/a 0 for which S...
-
Sketch out a molecular orbital energy diagram for CO and place the electrons in the levels appropriate for the ground state. The AO ionization energies are O2s: 32.3 eV; O2p: 15.8 eV; C2s: 19.4 eV;...
-
Cash from Operating Activities: ______________ Cash from Investing Activities: ______________ Cash from Financing Activities: ______________ Problem 2: Financial Ratios The GAP Macys 1 Current Ratio...
-
On January 1, 2021, Winky Enterprises issued 12% bonds dated January 1, 2021, with a face amount of $2,800,000. The bonds mature in 2030 (10 years). For bonds of similar risk and maturity, the market...
-
Using the following accounts and balances, prepare the stockholders' equity vection of the balance sheet. Pilty thousand shares of common stock are authorised, and 1,000 shares have been recoured,...

Study smarter with the SolutionInn App


