Predict the products of each of the following reactions and then balance each equation: (a) Mg(s) +
Question:
Predict the products of each of the following reactions and then balance each equation: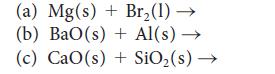
Transcribed Image Text:
(a) Mg(s) + Br₂(1)→ (b) BaO(s) + Al(s)→ (c) CaO(s) + SiO₂ (s) →
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Lets predict the products and balance the chemical equa...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Predict the products of each of the following reactions and then balance each equation: (a) Mg (OH) + HCl(aq) (b) Ca(s) + HO(1) (c) BaCO3(s) 4
-
Predict the products of each of the following reactions. (a) (b) (c) (d) OH Cl pyridine OH (1) NaH (2) CH2l HBr OH HNOg, H2SO4 H3C
-
Predict the major products of each of the following reactions and then balance the equations: (a) FeCrO4(s) + C(s) A (b) CrO (s) + HO*(aq) (c) MnO (s) + Al(s) A
-
If a restaurant's total sales on a given day were $4,350.00, and the restaurant had served 365 customers, what is the average dollar sale? $10.05 $11.92 $11.35 $11.05
-
Consider the following three stocks: a. Stock A is expected to provide a dividend of $10 a share forever. b. Stock B is expected to pay a dividend of $5 next year. Thereafter, dividend growth is...
-
Determine a direct form realization for the following linear phasefilters. (a) h(n) = (1.2, 3, 4.3, 2. 1) (b) h(n) = (1.2,3, 3, 2. 1}
-
Create a Windows Forms application. Use the following names for the project and solution, respectively: Gas Prices Project and Gas Prices Solution. Save the application in the VB2017\Chap08 folder....
-
Arun Company has had poor operating results for the past two years. As the accountant for Arun Company, you have the following information available to you: Total assets and owner's equity at the...
-
High Country, Inc., produces and sells many recreational products. The company has just opened a new plant to produce a folding camp cot that will be marketed throughout the United States. The...
-
Arrange the following atoms in order of increasing first ionization energy: boron, thallium, gallium.
-
Write the chemical equation for the reaction between (a) Cesium and oxygen (cesium reacts with oxygen in the same way as potassium); (b) Sodium oxide and water; (c) Lithium and hydrochloric acid; (d)...
-
Determine the constant speed at which the cable at A must be drawn in by the motor in order to hoist the load 6 m in 1.5 s. A D C B
-
Most research indicates that good leaders exhibit these leadership skills / https://emeritus.org/blog/leadership-skills-for-managers/ Which of these skills, in your opinion, are the most difficult to...
-
Consider the following account balances (in thousands) for the Shaker Corporation In the Dec 31.2021 Cash $200,000 and Capital $2,000,000 and Retained earnings $1,500,000 The balances of raw...
-
Given: a = -7,b=-519, c = < 5,-1,9 >,d= 2j - 4k, e = < 4, -6, -3> F = 6 -[312].G=124 -91 2x1 Determine the following if possible and if not possible explain why not. i. a ii. |c| iii. |F| iv. V. F-1...
-
I have been identified and approached by leaders who saw my potential and asked me to apply for a position. I was humbled and honored to be identified and I accepted the invitation. It has led to...
-
the object is 2.0mm?there are two converging lens on the right side of the object?one is 9.9cm far away from the object and has a focal point 9.0cm?the other is 101.1cm far away from the first lens...
-
With a single explanatory variable, the equation used to obtain the between estimator is where the overbar represents the average over time. We can assume that E(a.) = 0 because we have included an...
-
A heat engine has a heat input of 3 Ã 104 Btu/h and a thermal efficiency of 40 percent. Calculate the power it will produce, in hp. Source 3 x 10 Btu/h 40% HE Sink
-
How do the values of the AO coefficients in a MO differ for a delocalized and a localized bond?
-
What experimental evidence can you cite in support of the hypothesis that the electronegativity of a hybridized atom increases with increasing s-character?
-
Explain why all possible wave functions between the fully bonding and the fully anti-bonding are possible for the bands shown in Figure 24.22. Figure 24.22 N atomic orbitals 48 3.22B 28 2 atoms, N...
-
business law A partner may actively compete with the partnership True False
-
A company provided the following data: Selling price per unit $80 Variable cost per unit $45 Total fixed costs $490,000 How many units must be sold to earn a profit of $122,500?
-
Suppose a 10-year, 10%, semiannual coupon bond with a par value of $1,000 is currently selling for $1,365.20, producing a nominal yield to maturity of 7.5%. However, it can be called after 4 years...

Study smarter with the SolutionInn App


