Predict the standard potential of each of the following galvanic cells: 3+ (a) Pt(s)| Fe+ (aq), Fe+
Question:
Predict the standard potential of each of the following galvanic cells: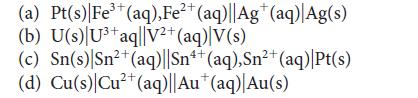
Transcribed Image Text:
3+ (a) Pt(s)| Fe³+ (aq), Fe²+ (aq)||Ag* (aq) Ag(s) (b) U(s) U³+ aq||V²+ (aq) V(s) 2+ (c) Sn(s) Sn²+ (aq)||Sn4+ (aq),Sn²+ (aq)|Pt(s) 2+ (d) Cu(s) Cu²+ (aq)||Au* (aq)| Au(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
cell To predict the standard cell potential El for each galvanic cell we can use the standard reduct...View the full answer

Answered By

Mwangi Clement
I am a tried and tested custom essay writer with over five years of excellent essay writing. In my years as a custom essay writer, I have completed more than 2,000 custom essays in a diverse set of subjects. When you order essays from me, you are working with one of the best paper writers on the web. One of the most common questions I get from customers is: “can you write my essay?” Upon hearing that request, my goal is to provide the best essays and overall essay help available on the web. I have worked on papers in subjects such as Nursing and Healthcare, English Literature, Sociology, Philosophy, Psychology, Education, Religious Studies, Business, Biological Sciences, Communications and Media, Physical Sciences, Marketing and many others. In these fields, my specialties lie in crafting professional standard custom writings. These include, but are not limited to: research papers, coursework, assignments, term papers, capstone papers, reviews, summaries, critiques, proofreading and editing, and any other college essays.
My extensive custom writings experience has equipped me with a set of skills, research abilities and a broad knowledge base that allows me to navigate diverse paper requirements while keeping my promise of quality. Furthermore, I have also garnered excellent mastery of paper formatting, grammar, and other relevant elements. When a customer asks me to write their essay, I will do my best to provide the best essay writing service possible. I have satisfactorily offered my essay writing services for High School, Diploma, Bachelors, Masters and Ph.D. clients.
I believe quality, affordability, flexibility, and punctuality are the principal reasons as to why I have risen among the best writers on this platform. I deliver 100% original papers that pass all plagiarism check tests (Turnitin, Copyscape, etc.). My rates for all papers are relatively affordable to ensure my clients get quality essay writing services at reasonable prices.
4.50+
5+ Reviews
14+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Predict the standard potential of each of the following galvanic cells: 3+ (a) Pt(s) Cr+ (aq), Cr+ (aq)||Cu+ (aq) |Cu(s) (b) Ag(s) AgI(s) I (aq)||CI (aq)|AgCl(s) Ag(s) (c) Hg(1) HgCl (s) CIT(aq) |Hg+...
-
A galvanic cell has the following cell reaction: M(s) + 2 Zn 2+ (aq) 2 Zn(s) + M 4+ (aq). The standard potential of the cell is 10.16 V. What is the standard potential of the M 4+ /M redox couple?
-
Sketch the galvanic cells based on the following half-reactions. Calculate Ïo, show the direction of electron flow and the direction of ion migration through the salt bridge, identify the...
-
Built-Tite uses job order costing. The T-account below summarizes Factory overhead activity for the current year. Factory Overhead Debit Credit 16,200 106,600 25,200 60,200 1. Compute total applied...
-
Weighted-average method, inspection at 80% completion (chapter appendix). (A. Atkinson) The Kim Company is a furniture manufacturer with two departments: molding and finishing. The company uses the...
-
As the situation of Exercise 2.73 might suggest, statistical procedures are often used for control of quality (i.e., industrial quality control). At times, the weight of a product is an important...
-
Understand risk and the importance of good project risk management? LO.1
-
Relevant-cost approach to pricing decisions. Stardom, Inc., cans peaches for sale to food distributors. All costs are classified as either manufacturing or marketing. Stardom prepares monthly...
-
DreamHome operates in a number of countries and sells a wide variety of home goods, from garden furniture to kitchenware, to achieve continuous growth. From this data, we can conclude that DreamHome...
-
As a financial analyst at Glencolin International (GI) you have been asked to revisit your analysis of the two capital investment alternatives submitted by the production department of the firm....
-
A careless laboratory technician prepares 300.0 mL of 0.0175 m KOH(aq) and pipets 25.0 mL of the solution into a beaker. The beaker is allowed to stand in a warm place for two days before use, during...
-
The molar solubility of silver sulfite, Ag 2 SO 3 , is 1.55 * 10 5 mol L 1 . What is the K sp of silver sulfite?
-
A currently owned shredder originally costing \($800,000\) was purchased 6 years ago for use in a refuse-powered electrical generating plant. It was depreciated as MACRS-GDS 5-year property due to...
-
10.1 Learning Outcomes: Describe managers' appropriate use of power and influence. Identify traits and characteristics of successful leaders. Identify behaviors of successful leaders 10.2 Action...
-
Suppose Ron went to Rio de Janeiro in 2019 when the dollar was worth 4 reals. The price of a cup of coffee at Starbucks in the US was $4, so when Ron converted $4 into reals he had 16 reals, which...
-
The expected annual net income is $200,000; the average investment is $800,000; and depreciation expense is $50,000. Calculate the annual rate of return.
-
How do you define humanities?
-
Write the types of partners ?
-
In Exercise 5.2.10, the answer to part (b) was larger than the answer to part (a). Argue that this must necessarily be true, no matter what the population mean id standard deviation might be.
-
Assume that your audit team has established the following parameters for the examination of ELM's sales transactions: LO G-3 Risk of incorrect acceptance...
-
Determine the half-cell reactions and the overall cell reaction, calculate the cell potential, and determine the equilibrium constant at 298.15 K for the cell Is the cell reaction spontaneous as...
-
Consider the half-cell reaction AgCl(s) + e Ag(s) + Cl (aq). If (AgCl, s) = 109.71 kJ mol 1 , and if E = +0.222 V for this half-cell, calculate the standard Gibbs energy of formation of Cl (aq).
-
For a given overall cell reaction, S o R = 16.5 J mol -1 K -1 and H o R = 270.0 kJ mol -1 . Calculate E o and (E o /t) P . Assume that n = 2.
-
How do warehouses and distribution centers differ? What is cross-docking and why might a company choose to cross-dock a product? What kinds of products can be delivered electronically? What kinds...
-
Strawberry Inc. has historically been an all-equity firm. The analyst expects EBIT to be $1.5B in perpetuity starting one year from now. The cost of equity for the company is 11.5% and the tax rate...
-
Guzman company received a 60- day, 5 % note for 54,000 dated July 12 from a customer on account. Determine the due date on note. Determine the maturity value of the note and journalize the entry of...

Study smarter with the SolutionInn App


