Predict the standard potential of each of the following galvanic cells: 3+ (a) Pt(s) Cr+ (aq), Cr+
Question:
Predict the standard potential of each of the following galvanic cells: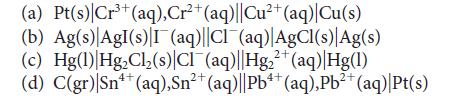
Transcribed Image Text:
3+ (a) Pt(s) Cr³+ (aq), Cr²+ (aq)||Cu²+ (aq) |Cu(s) (b) Ag(s) AgI(s) I (aq)||CI (aq)|AgCl(s) Ag(s) (c) Hg(1) Hg₂Cl₂ (s) CIT(aq) |Hg₂+ (aq) Hg(1) 2+ 4+ (d) C(gr) |Sn¹+ (aq),Sn²+ (aq)||Pb4+ (aq), Pb²+ (aq)|Pt(s) 2+
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a 075 ...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Predict the standard potential of each of the following galvanic cells: 3+ (a) Pt(s)| Fe+ (aq), Fe+ (aq)||Ag* (aq) Ag(s) (b) U(s) U+ aq||V+ (aq) V(s) 2+ (c) Sn(s) Sn+ (aq)||Sn4+ (aq),Sn+ (aq)|Pt(s)...
-
A galvanic cell has the following cell reaction: M(s) + 2 Zn 2+ (aq) 2 Zn(s) + M 4+ (aq). The standard potential of the cell is 10.16 V. What is the standard potential of the M 4+ /M redox couple?
-
Sketch the galvanic cells based on the following half-reactions. Calculate Ïo, show the direction of electron flow and the direction of ion migration through the salt bridge, identify the...
-
Solve: y(4) +18y"+81y = 0 y(0) = -4, y'(0) = 8, y'(0) = 42, y"(0) = -108 - Submit Question X
-
Weighted-average method, spoilage. Appleton Company makes wooden toys in its Forming Department, and it uses the weighted-average method of process costing. All direct materials are added at the...
-
The grades of a class of 9 students on a midterm report (x) and on the final examination (y) are as follows(a) Estimate the linear regression line.(b) Estimate the final examination grade of a...
-
Your project team has decided not to use an upcoming release of software because it might cause your schedule to slip. Which negative risk response strategy are you using? a. avoidance b. acceptance...
-
Kiwi Charter Corp. reported $1,612,530 of net income for 2014. On November 2, 2014, it declared and paid the annual preferred dividends of $234,000. On January 1, 2014, Kiwi had 80,000 and 270,000...
-
[The following information applies to the questions displayed below.] Wallys Widget Company (WWC) incorporated near the end of 2011. Operations began in January of 2012. WWC prepares adjusting...
-
Describe how to apply stratified sampling to sample from the Credit Risk Data file based on the different types of loans. Implement your process in Excel to choose a random sample consisting of 10%...
-
Carbonate and hydrogen carbonate (bicarbonate) ions contribute to buffering in a variety of natural systems. You are investigating their role in groundwater percolating through limestone hills into a...
-
Estimate the enthalpy of deprotonation of formic acid at 25C, given that K a = 1.765 * 10 4 at 20C and 1.768 * 10 4 at 30C.
-
Studies show that when environmental factors impose an upper bound on the possible size of a population P(t), the population often tends to grow in such a way that the percentage rate of change of...
-
Carol's Cupcakes has grown from a home business into a one of the largest event and wedding catering companies in the area. Its founder, Carol Thompson, first dreamed of owning her own company while...
-
Many things have changed for businesses in 2022. The previous 2 business years of 2020 and 2021 have tested businesses and the workforce like nothing else. Not only were profits reduced, and...
-
1) Virginia Tech's motto is "Ut Prosim" which means 'That I May Serve'. Share how you contribute to a community that is important to you. How long have you been involved? What have you learned and...
-
Person Is Arianna Grande Answer all questions Who are they? How successful are they? Why would companies be interested in partnering with them? Identify one company from their industry that you feel...
-
Imagine you have just retired after a long and very successful career (as a physiotherapist). Congratulations! You've made such an impact in the world that business and community leaders from around...
-
Refer to the heart rate distribution of Exercise 4.S.16. Suppose we take a random sample of size 400 from this distribution. How many observations do we expect to obtain that fall between 0 and 15?...
-
As economic conditions change, how do banks adjust their asset portfolio?
-
The following data were observed in an experiment on the photoelectric effect from potassium: Graphically evaluate these data to obtain values for the work function and Plancks constant. 1019 Kinetic...
-
The power (energy per unit time) radiated by a blackbody per unit area of surface expressed in units of W m 2 is given by P = T 4 with = 5.67 10 8 W m 2 K 4 . The radius of the sun is 7.00 10 5...
-
The work function of palladium is 5.22 eV. What is the minimum frequency of light required to observe the photoelectric effect on Pd? If light with a 200. nm wavelength is absorbed by the surface,...
-
Which of the following statements regarding traditional cost accounting systems is false? a. Products are often over or under cost in traditional cost accounting systems. b. Most traditional cost...
-
Bart is a college student. Since his plan is to get a job immediately after graduation, he determines that he will need about $250,000 in life insurance to provide for his future wife and children...
-
Reporting Financial Statement Effects of Bond Transactions (please show me how you got the answers) Lundholm, Inc., which reports financial statements each December 31, is authorized to issue...

Study smarter with the SolutionInn App


