Predict whether each of the following equilibria will shift toward products or reactants with a temperature increase:
Question:
Predict whether each of the following equilibria will shift toward products or reactants with a temperature increase: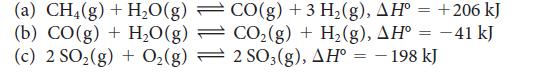
Transcribed Image Text:
(a) CH4(g) + H₂O(g) (b) CO(g) + H₂O(g) (c) 2 SO₂(g) + O₂(g) CO(g) +3 H₂(g), AH° = +206 kJ CO₂(g) + H₂(g), AH° = −41 kJ 2 SO3(g), AH° = - 198 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
To predict the direction of shift for each equilibrium with a temperature increase we need to determ...View the full answer

Answered By

Anurag Agrawal
I am a highly enthusiastic person who likes to explain concepts in simplified language. Be it in my job role as a manager of 4 people or when I used to take classes for specially able kids at our university. I did this continuously for 3 years and my god, that was so fulfilling. Sometimes I've skipped my own classes just to teach these kids and help them get their fair share of opportunities, which they would have missed out on. This was the key driver for me during that time. But since I've joined my job I wasn't able to make time for my passion of teaching due to hectic schedules. But now I've made a commitment to teach for at least an hour a day.
I am highly proficient in school level math and science and reasonably good for college level. In addition to this I am especially interested in courses related to finance and economics. In quest to learn I recently gave the CFA level 1 in Dec 19, hopefully I'll clear it. Finger's crossed :)
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Predict whether each of the following equilibria will shift toward products or reactants with a temperature increase: 2 NO(g), AH = +57 kJ 2 X(g), where X is a halogen (a) NO4(g) (b) X(g) (c) Ni(s)...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Predict whether each of the following nuclides is stable or unstable (radioactive). If the nuclide is unstable, predict rhe type of radioactivity you would expect it to exhibit. a. 4519K b. 5626Fe c....
-
Use f(x) and g(x) to find a formula for each expression. Identify its domain. (a) (f + g)(x) (c) (fg)(x) (b) (f- g)(x) (d) (f/g)(x)
-
A wire of cross-sectional area A, length L 1 , resistivity 1 , and temperature coefficient 1 is connected end to end to a second wire of the same cross-sectional area, length L 2 , resistivity 2 ,...
-
What is an explanation for the fact, experimentally proven, that quartz sand grains are rounded much faster by wind than by a river?
-
Jennifer Capriati, Inc. issued a $100,000, 4-year, 11% note at face value to Forest Hills Bank on January 1, 2008, and received $100,000 cash. The note requires annual interest payments each December...
-
A simplified representation for cooling in very large-scale integration (VLSI) of microelectronics is shown in the sketch. A silicon chip is mounted in a dielectric substrate, and one surface of the...
-
Suppose we have the following returns for large - company stocks and Treasury bills over a six - year period: Year Large - Company stocks US Treasury bills 1 3 . 9 2 % 5 . 9 0 % 2 1 4 . 1 8 2 . 5 3 3...
-
One of the shops in hackerMall is offering discount coupons based on a puzzling porblem. There are n tags, where each tag has a value denoted by val [ [ i ] . ] . A customer needs to choose teh tags...
-
Hexane, C 6 H 14 , and cyclohexane, C 6 H 12 , form an ideal solution. The vapor pressure of hexane is 151 Torr and that of cyclohexane is 98 Torr at 25.0C. Calculate the total vapor pressure of each...
-
A solution prepared by adding 0.50 g of a polymer to 0.200 L of toluene (methylbenzene, a common solvent) showed an osmotic pressure of 0.582 Torr at 20 8C. What is the molar mass of the polymer?
-
Komissarov Company has a debt investment in the bonds issued by Keune Inc. The bonds were purchased at par for 400,000 and, at the end of 2015, have a remaining life of 3 years with annual interest...
-
Q Proprietorinc (the lessee) enters into a 10 year lease of a property with an option to extend the contract for 5 years. Lease payments are $50,000 per year, payable at the beginning of each year....
-
1.Think about your investment Possibility for 3 years holding period in real investment environment? A.What could be your investment objectives? B. What amount of fund you could invest for three...
-
3- The student council normally sells 1500 school T-shirts for $12 each. This year they plan to decrease the price of the T-shirts. Based on student feedback, they know that for every $0.50 decrease...
-
2. The notation {f(x): x S} means "the set of all values that can be produced by substituting an element x of set S into f(x)." For example, the set of all odd integers can be expressed as {2k+1kZ}....
-
Implementation guidance for IFRS 2 indicates that it "accompanies, but is not part of, IFRS 2." In other words, this implementation guidance is considered mandatory. integral to the standard. not...
-
A capillary rise experiment is proposed for a high school physics class. The students are told that for water in clean glass tubes, the contact angle between liquid and glass (0) is 90. The students...
-
Identify the source of funds within Micro Credit? How does this differ from traditional sources of financing? What internal and external governance mechanisms are in place in Micro Credit?
-
The amino acid glycine dimerizes to form the dipeptide glycylglycine according to the reaction 2Glycine(s) Glycylglycine(s) + H 2 O(l) Calculate ÎS, ÎSsurroundings , and ÎSuniverse...
-
Draw the major product expected from each of the following reactions: (a) (b) ? Lindlar's catalyst Pt -? Ni,B -? Ni
-
Draw the major product expected when each of the following alkynes is treated with sodium in liquid ammonia: (a) (b) (c) (d)
-
44. Dryer Companys policy is to keep 25% of the next month's sales in ending inventory. If Dryer meets its ending inventory policy at the end of April and sales are expected to be 24,000 units in May...
-
What general conclusions can you draw about your companys liquidity, solvency and productivity based on your ratio calculations. Working Capital 2017 = $9,994 M 2016 = $10,673 M Current Ratio 2017 =...
-
Tami Tyler opened Tami's Creations, Incorporated, a small manufacturing company, at the beginning of the year. Getting the company through its first quarter of operations placed a considerable strain...

Study smarter with the SolutionInn App


