Predict whether each of the following equilibria will shift toward products or reactants with a temperature increase:
Question:
Predict whether each of the following equilibria will shift toward products or reactants with a temperature increase: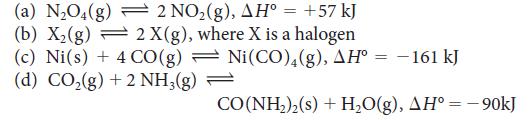
Transcribed Image Text:
2 NO₂(g), AH° = +57 kJ 2 X(g), where X is a halogen → (a) N₂O4(g) (b) X₂(g) (c) Ni(s) + 4CO(g) (d) CO₂(g) + 2 NH3(g) Ni(CO)4(g), AH = -161 kJ CO(NH₂)₂(s) + H₂O(g), AH° = - 90kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a and b are endothermic and raising ...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Predict whether each of the following equilibria will shift toward products or reactants with a temperature increase: (a) CH4(g) + HO(g) (b) CO(g) + HO(g) (c) 2 SO(g) + O(g) CO(g) +3 H(g), AH = +206...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Predict whether each of the following nuclides is stable or unstable (radioactive). If the nuclide is unstable, predict rhe type of radioactivity you would expect it to exhibit. a. 4519K b. 5626Fe c....
-
Factor each polynomial. 125k - 64k 4
-
The space between two metallic coaxial cylinders of length L and radii a and b is completely filled with a material having a resistivity . (a) What is the resistance between the two cylinders? (b)...
-
What do we mean when we say that accounting is a means rather than an end?
-
(a) In a troubled debt situation, why might the creditor grant concessions to the debtor? (b) What type of concessions might a creditor grant the debtor in a troubled debt situation? (c) What is...
-
Juan Companys output for the current period was assigned a $ 150,000 standard direct materials cost. The direct materials variances included a $ 12,000 favorable price variance and a $ 2,000...
-
TASK 2 : Calculate Premium and Claim value form the given data for the Starbuds Insurance Company for the year 2019. A. Premium Earned B. Claims Incurred Starbuds General Insurance SAOG submits the...
-
1. How would you describe Danielle Oviedo's approach to leadership? 2. What would you predict about Danielle's future success as a leader? Why? 3. In what ways, if any, does Danielle function as a...
-
(a) Calculate the mass of CaCl 2 6H 2 O needed to prepare 0.125 m CaCl 2 (aq) by using 500. g of water. (b) What mass of NiSO 4 6H 2 O must be dissolved in 500. g of water to produce 0.22 m NiSO 4...
-
Write the reaction quotient Q for (a) 2 BCl3(g) + 2 Hg(1) BCl(s) + HgCl(s) (b) P4S10(s) + 16 HO(1) 4 H3PO4(aq) + 10 HS(aq) (c) Br(g) + 3 F(g) 2 BrF3(g)
-
Gomez Corporation uses straight-line depreciation for financial reporting purposes but an accelerated method for tax purposes. Is it acceptable to use different methods for the two purposes? What is...
-
a) Provide a brief background of the Honda Motor company and industry, then identify a current ethical issue that has an effect on the industry that the Honda Motor company is operating in. 1b)...
-
If you own, did you consider leasing? If yes, why did you choose a purchase over a lease? If you lease, why did you go with a lease? List the specific advantages you feel you gained by leasing. If...
-
If 5 people worked in a process for 8 hours, which included a 0.5 hour break, and they produced 900 units. What was the worker hours per unit? from below: 2.7 minutes 24 minutes 2.5 minutes 22.5...
-
12. Access the following PIDS using the scan tool and verify current input signal status and record the status below? PIDS APP1 APP2 ECT IAT MAF RPM TP1 TP2 VSS Signal Status WSM Specifications
-
With Twitter being on the verge of bankruptcy and undergoing mass resignation, what are some creative and relative directions managers of the social media platform could take to improve the brand?
-
Determine the force required to drag a small barge (10 ft by 30 ft) in a shallow canal (3 inches deep) in order to maintain a velocity of 5 ft/sec. Assume that the fluid is behaving in a Newtonian...
-
In a system with light damping (c < cc), the period of vibration is commonly defined as the time interval d = 2/d corresponding to two successive points where the displacement-time curve touches one...
-
Is the following statement correct? Because dry ice sublimes, carbon dioxide has no liquid phase. Explain your answer.
-
For the equation of state V m = RT /P + B(T), show that ' () = -T- d?
-
The following data are a DSC scan of a solution of a T4 lysozyme mutant. From the data determine T m . Determine also the excess heat capacity ÎC P at T = 308 K. Determine also the intrinsic...
-
Given that rJ = 6.3%, rRF = 4.1%, and rM = 9.4%, determine the beta coefficient for Stock J that is consistent with equilibrium.
-
Simon Companys year-end balance sheets follow. At December 31 2017 2016 2015 Assets Cash $ 33,019 $ 37,839 $ 38,623 Accounts receivable, net 93,822 65,556 54,152 Merchandise inventory 117,963 89,253...
-
PLEASE REFER TO THE 2018 ANNUAL REPORT OF STARBUKS FOR THE YEAR FISCAL YR 2018, ENDING SEPTEMBER 30, 2018. Refer to the management discussion & analysis section and write a one page summary...

Study smarter with the SolutionInn App


