Write the reaction quotient Q for (a) 2 BCl3(g) + 2 Hg(1) BCl(s) + HgCl(s) (b) P4S10(s)
Question:
Write the reaction quotient Q for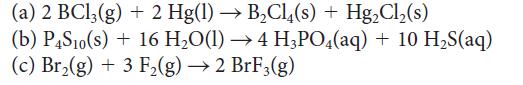
Transcribed Image Text:
(a) 2 BCl3(g) + 2 Hg(1)→ B₂Cl(s) + Hg₂Cl₂(s) (b) P4S10(s) + 16 H₂O(1)→ 4 H3PO4(aq) + 10 H₂S(aq) (c) Br₂(g) + 3 F₂(g) →2 BrF3(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
1 a 2 b ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
How would you write the reaction quotient expression for Cu(s) + 2 H + (aq) Cu 2+ (aq) + H 2 (g) ? Write this first in terms of activities and then convert to pressures and concentrations.
-
The oxidation of SO 2 to SO 3 is one of the reactions involved in the formation of acid rain. If you want to predict the spontaneous direction of the reaction for a specific mixture of the gases, you...
-
Refer to Example 9. After how many seconds will the rocket be (a) 240 ft above the ground? (b) 112 ft above the ground? Data from in Example 9 EXAMPLE 9 Using a Quadratic Function in an Application...
-
The space between two concentric spherical-shell conductors is filled with a material that has a resistivity of 109 m. If the inner shell has a radius of 1.5 cm and the outer shell has a radius of...
-
What is the primary distinction between financial accounting and managerial accounting?
-
What are take-or-pay contracts and through-put contracts?
-
Petal Providers Corporation, described in Problem 5, is interested in estimating its sustainable sales growth rate. Last year, revenues were $1 million; net profit was $50,000; investment in assets...
-
We are evaluating a project that costs $101256, has a seven-year life, and has no salvage value. Assume that depreciation is straight-line to zero over the life of the project. Sales are projected at...
-
Categorize each of the following characteristics as being more representative of either traditional manufacturing or lean production. 1. Quality tends to be inspected-in rather than built-in. 2....
-
Predict whether each of the following equilibria will shift toward products or reactants with a temperature increase: 2 NO(g), AH = +57 kJ 2 X(g), where X is a halogen (a) NO4(g) (b) X(g) (c) Ni(s)...
-
Benzene, C 6 H 6 , and toluene, C 6 H 5 CH 3 , form an ideal solution. The vapor pressure of benzene is 94.6 Torr and that of toluene is 29.1 Torr at 25C. Calculate the total vapor pressure of each...
-
What advice would you give to the creator of the following graph? Consider the basic guidelines for a clear graph, for avoiding chartjunk, and regarding the ways to mislead through statistics. Give...
-
The relevance ( Relevance ) and the reliability ( Reliability ) represent two characters Key qualitative statistics of information n accountant. What What do these two mean? terms in an accounting...
-
A virtual memory system has a page size of 1024 bytes, six virtual pages, and five physical page frames. The page table is shown in Table Q2(d) as follows: Virtual Page Number (VPN) 0 Page Frame...
-
31. z = x + 2xy, determine which of (I)-(II) in Figure 12.31 are cross- sections with x fixed and which are cross-sections with y fixed. (1) (11) -2 -2+
-
WHAT DOES SOCIETY EXPECT FROM ORGANIZATIONS AND MANAGERS? Introduction: TOMS Shoes has a unique idea to promote corporate social responsibility. For each pair of shoes it sells, it donates a pair to...
-
1. A car heading east turns right at a corner. The car turns at a constant speed of 20.0 m/s. After 12 s, the car completes the turn, so that it is heading due south at 20.0 m/s. Calculate the car's...
-
Establish the equivalence between (a) Absolute viscosity units of poise and lb sec/ft2 (b) Kinematic viscosity units of stoke and the British system unit.
-
1. True or False. Pitfalls to consider in a statistical test include nonrandom samples, small sample size, and lack of causal links. 2. Because 25 percent of the students in my morning statistics...
-
Make a drawing indicating the four-step process d of Figure 8.4 in Figure 8.13. Figure 8.4 Figure 8.13 Critical- point Liquid Solid Triple point Gas Tm Temperature Pressure/bar Critical point P....
-
Dogs cool off in hot weather by panting. Write a chemical equation to describe this process and calculate H o R.
-
Use the following data at 298.15 K to complete this problem: Calculate ÎH o R for a. OH(g) H(g) + O(g) b. H 2 O(g) 2H(g) + O(g) c. H 2 O(g) H(g) + OH(g) Assuming ideal gas behavior, calculate...
-
Columbus Industries makes a product that sells for $37 a unit. The product has a $29 per unit variable cost and total fixed costs of $10,000. At budgeted sales of 1,950 units, the margin of safety...
-
18. Suppose that Maxima shares are selling for $10 per share and you own a call option to buy Maxima shares at $7.50. The intrinsic value of your option is:
-
ABC Insurance Company reported the following information on its accounting statements last year: What was ABC 's expense ratio last year

Study smarter with the SolutionInn App


