Question: Use the following data at 298.15 K to complete this problem: Calculate ÎH o R for a. OH(g) H(g) + O(g) b. H 2 O(g)
Use the following data at 298.15 K to complete this problem:
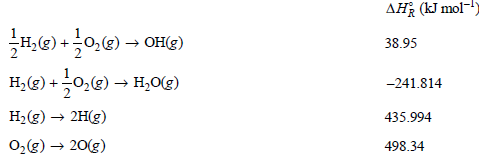
Calculate ΔHoR for
a. OH(g) †’ H(g) + O(g)
b. H2O(g) †’ 2H(g) + O(g)
c. H2O(g) †’ H(g) + OH(g)
Assuming ideal gas behavior, calculate ΔHoR and ΔUoR for all three reactions.
AH (kJ mol- H,(g) +0,(e) OH() H, (g) +0,(g) H,0(g) 38.95 -241.814 H2(g) 2H(g) 0,g) 20(g) 435.994 498.34
Step by Step Solution
3.36 Rating (171 Votes )
There are 3 Steps involved in it
a U o R H o R nRT 42822 kJ mol 1 8314 J mol 1 K 1 29815 K 42574 kJ m... View full answer

Get step-by-step solutions from verified subject matter experts


