Predict which of the hydrocarbons below has the greater standard molar entropy at 25C and 1 bar.
Question:
Predict which of the hydrocarbons below has the greater standard molar entropy at 25°C and 1 bar. Explain your reasoning.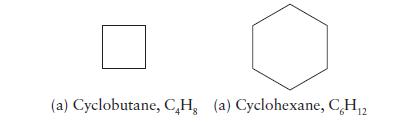
Transcribed Image Text:
(a) Cyclobutane, CH, (a) Cyclohexane, C,H2 12
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Cyclohexane has m...View the full answer

Answered By

Issa Shikuku
I have vast experience of four years in academic and content writing with quality understanding of APA, MLA, Harvard and Chicago formats. I am a dedicated tutor willing to hep prepare outlines, drafts or find sources in every way possible. I strive to make sure my clients follow assignment instructions and meet the rubric criteria by undertaking extensive research to develop perfect drafts and outlines. I do this by ensuring that i am always punctual and deliver quality work.
5.00+
6+ Reviews
13+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The standard molar entropy of NHJ (g) is 192.45 T K-I mol-1 at 298 K, and its heat capacity is given by eqn 2.25 with the coefficients given in Table 2.2. Calculate the standard molar entropy at (a)...
-
Estimate the standard enthalpies of formation at 25 C and 1 bar of (a) OH(g); (b) N 2 H 4 (g). Write Lewis structures and use data from Table 10.3, as necessary. Table 10.3 TABLE 10.3 Some Average...
-
Predict which of the following liquids has greater surface tension: ethanol (C2H5OH) or dimethyl ether (CH3OCH3)?
-
You are deciding between two mutually exclusive investment opportunities. Both require the same initial investment of $10 million. Investment A will generate $2 million per year (starting at the end...
-
Your roommate asks your help in understanding the major steps in the flow of costs in a job order cost system. Identify the steps for your roommate.
-
The given curve is rotated about the y-axis. Find the area of the resulting surface. x 2/3 + y 2/3 = 1, 0 y 1
-
A company reports net income of $75,000. Its weightedaverage common shares outstanding is 19,000. It has no other stock outstanding. Its earnings per share is: a. $4.69 b. $3.95 c. $3.75 d. $2.08 e....
-
When Ridge Intellectual, a graphics design firm, implemented 360-degree feedback in its organization two years ago, it was met with resistance and was eventually discontinued. Ron Bartlett, the...
-
Nicolette is a self-employed consultant who uses 20% of her residence as an office. The office is used exclusively for business and is frequented by customers on a regular basis. Nicolette also uses...
-
A piston confines 0.250 mol He (g) in 1.50 L at 25C. Two experiments are performed. (a) The gas is allowed to expand through an additional 1.00 L against a constant pressure of 2.00 atm. (b) The gas...
-
Considering positional disorder, would you expect a crystal of octahedral cis-MX 2 Y 4 to have the same, higher, or lower residual entropy than the corresponding trans isomer? Explain your...
-
Which of the following is not one of the three broad categories of self-leadership? (a) constructive-thought-pattern strategies (b) natural-reward (c) behavior-focused (d) achievement-focus
-
The balances of selected accounts of Casper Company on February 28, 20X1, were as follows: Sales $250,000 and Sales Returns and Allowances $4,000. The firm's net sales are subject to an 7 percent...
-
1. Draw and label force diagrams for the physics book and for the calculator. Add equality marks showing any equalities between force diagrams. Circle and label any Newton's third law pairs. (6 pts)...
-
Consider the Lincoln Tunnel, which was built in 1939 under the Hudson River in New York. Assume the tunnel to be empty with perfectly conducting walls and rectangular cross section with width 6.55 m...
-
Examine a well-known principal-agent contract, the sale of your home by a licensed realtor. You will use the following data to analyze this case. Your home is the typical home, approximately 1,875 sq...
-
i) Generate a third degree polynomial in x and y named g(x, y) that is based on your mobile number (Note: In case there is a 0 in one of the digits replace it by 3). Suppose your mobile number is...
-
Experimentally, how do you think researchers were able to determine that the Y chromosome causes maleness in mammals, whereas the ratio of X chromosomes to the sets of autosomes causes sex...
-
As you rewrite these sentences, replace the cliches and buzzwords with plain language (if you don't recognize any of these terms, you can find definitions online): a. Being a jack-of-all-trades, Dave...
-
Calculate the change in entropy that occurs when 18.02 g of ice at 210.0oC is placed in 54.05 g of water at 100.0oC in a perfectly insulated vessel. Assume that the molar heat capacities for H2O(s)...
-
The synthesis of glucose directly from CO2 and H2O and the synthesis of proteins directly from amino acids are both non-spontaneous processes under standard conditions. Yet these processes must occur...
-
A green plant synthesizes glucose by photosynthesis as shown in the reaction 6CO2(g) + 6H2O(l) C6H12O6(s) + 6O2(g) Animals use glucose as a source of energy: C6H12O6(s) + 6O2(g) 6CO2(g) + 6H2O(l)...
-
Create a Data Table to depict the future value when you vary the interest rate and the investment amount. Use the following assumptions: Interest Rates: Investment Amounts:-10.0% $10,000.00 -8.0%...
-
Isaac earns a base salary of $1250 per month and a graduated commission of 0.4% on the first $100,000 of sales, and 0.5% on sales over $100,000. Last month, Isaac's gross salary was $2025. What were...
-
Calculate the price, including both GST and PST, that an individual will pay for a car sold for $26,995.00 in Manitoba. (Assume GST = 5% and PST = 8%) a$29,154.60 b$30,234.40 c$30,504.35 d$28,334.75...

Study smarter with the SolutionInn App


