Recognizing the monomer units in polymers is important for understanding how the polymers are prepared. Suppose you
Question:
Recognizing the monomer units in polymers is important for understanding how the polymers are prepared. Suppose you are studying the properties of various polymer materials and want to relate their properties to the building blocks used in their synthesis. Write the formulas of
(a) The monomers of Kevlar, a strong fiber used to make bulletproof vests,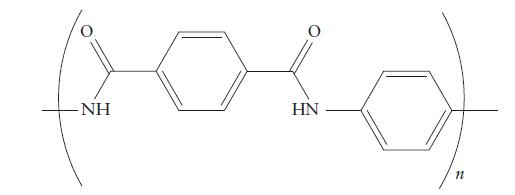
and
(b) Two repeating units of the polymer that is formed when peroxides are added to CH3CH2CH=CH2 at a high temperature and pressure.
PLAN (a) Look at the backbone of the polymer, the long chain to which the other groups are attached. If the atoms are all carbon atoms, then the compound is an addition polymer. If ester groups are present in the backbone, then the polymer is a polyester and the monomers will be an acid and an alcohol. If the backbone contains amide groups, then the polymer is a polyamide and the monomers will be an acid and an amine. (b) If the monomer is an alkene or alkyne, then the monomers will add to one another; a π-bond will be replaced by new σ-bonds between the monomers. If the monomers consist of an acid and an alcohol or amine, then a condensation polymer forms with the loss of a molecule of water.
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





