State whether reactants or products will be favored by an increase in the total pressure (resulting from
Question:
State whether reactants or products will be favored by an increase in the total pressure (resulting from compression) on each of the following equilibria. If there is no change, explain why that is so.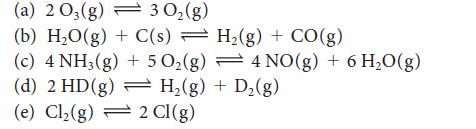
Transcribed Image Text:
(a) 203(g) (b) H₂O(g) + C(s) (c) 4 NH3(g) + 5 O₂(g) (d) 2 HD (g) (e) Cl₂(g) 30₂(g) H₂(g) + CO(g) 2 Cl(g) 4 NO(g) + 6H₂O(g) H₂(g) + D₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a Reactants ...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
1. In the classical model, it is thought that the long-run: A. and short-run aggregate supply curves are both upward sloping. B. aggregate supply curve is vertical and the short-run aggregate supply...
-
In Problems 5994, solve each inequality. Express your answer using set notation or interval notation. Graph the solution set. 2x2 3 + x
-
The Prism gas permeation process developed by the Monsanto Company is highly selective for hydrogen when using hollow-fiber membranes of materials such as silicone-coated polysulphone. In a typical...
-
A mine hoist uses a 2-in 6 x 19 monitor-steel wire rope. The rope is used to haul loads of 4 tons from the shaft 480 ft deep. The drum has a diameter of 6 ft, the sheaves are of good-quality cast...
-
(e) Produce a figure similar to Figure 1.4, but with the line of best fit from the mixed model instead of the simple regression line. Add the shrunk means to this plot. Comment on their distribution...
-
In a recent balance sheet, Microsoft Corporation reported Property, Plant, and Equipment of $15,082 million and Accumulated Depreciation of $7,547 million. a. What was the book value of the fixed...
-
AJ enters Baylor as a freshman, planning to major in accounting. On this same day he signs a five year, 10% note payable to a local car dealer for a $40,000 model Jeep. Payments are to be made at the...
-
What is the value of CP's revised offer on December 8 (before CP "sweetened" its offer by adding the CVR security)? In your analysis, assume the following: a) A valuation date of December 31, 2015,...
-
Explain the effect that an increase in temperature has on each of the following properties: (a) Viscosity; (b) Surface tension; (c) Vapor pressure; (d) Evaporation rate.
-
Two unknown molecular compounds were being studied. A solution containing 5.00 g of compound A in 100. g of water froze at a lower temperature than a solution containing 5.00 g of compound B in 100....
-
Fill in the blank with an appropriate word, phrase, or symbol(s). In an experiment, if there is neither a gain nor a loss in the long run, the expected value is ________ .
-
A parent acquires all of the stock of a subsidiary for $40 million in cash. The subsidiarys books report the following account balances at the date of acquisition (in trial balance format)....
-
1. Given: The sign for the Inn of the Prancing Pony in Bree-yes, it comes in pints-is fixed on the end of a beam of length 5L. If the sigh deflects too much then Gandalf will hit his head when he...
-
Q21) Add positive and negative charges as shown in the diagram below. Use the arrows of the simulation to guide you in drawing continuous electric field lines around and in between the three charges....
-
When 10.1 g CaO is dropped into a styrofoam coffee cup containing 157 g H2O at 18.0C, the temperature rises to 35.8C. Calculate the enthalpy change of the following reaction in kJ/mol CaO. Assume...
-
4-12. Sometimes heterogeneous chemical reactions take place at the walls of tubes in which reactive mixtures are flowing. If species A is being consumed at a tube wall because of a chemical reaction,...
-
a) What is containment? b) Why is disconnection undesirable? c) What is black holing?
-
Question 2 For an n x n matrix A = form) via (aij)
-
By looking at the a and b values for the van der Waals equation of state, decide whether 1 mole of O 2 or H 2 O has the higher pressure at the same value of T and V.
-
In the absence of turbulent mixing, the partial pressure of each constituent of air would fall off with height above sea level in the Earths atmosphere as P i = P i 0 e -M,g/RT where P i is the...
-
Propose a mechanism for the following transformation: . 1) Excess LA, 2) H20
-
Kelley Enterprises In October 1989, Pat Kelley.wus in his office, preparing the 1990 budget and contemplating the recent races of his business. Orders had been plentiful Lately that he though that...
-
The MegaMart Company began 2024 with inventory of 15,000 units at a cost of $6 per unit. During 2024, 55,000 units were purchased for $8.00 each. Sales for the year totaled 61,500 units leaving 8,500...
-
How much would Juanita's monthly premium be for a 10-year term insurance policy with a face value of $260,000, based on Table 19-1 and Table 19-2 (in $)? She turned 24 years old on her last birthday....

Study smarter with the SolutionInn App


