Suppose the reference electrode for Table 6M.1 were the standard calomel electrode, Hg 2 Cl 2 /Hg,
Question:
Suppose the reference electrode for Table 6M.1 were the standard calomel electrode, Hg2Cl2/Hg, Cl2([Cl2] = 1.00 mol · L–1), with its E° set equal to 0. Under this system, what would be the potential for
(a) The standard hydrogen electrode;
(b) The standard Cu2+/Cu redox couple?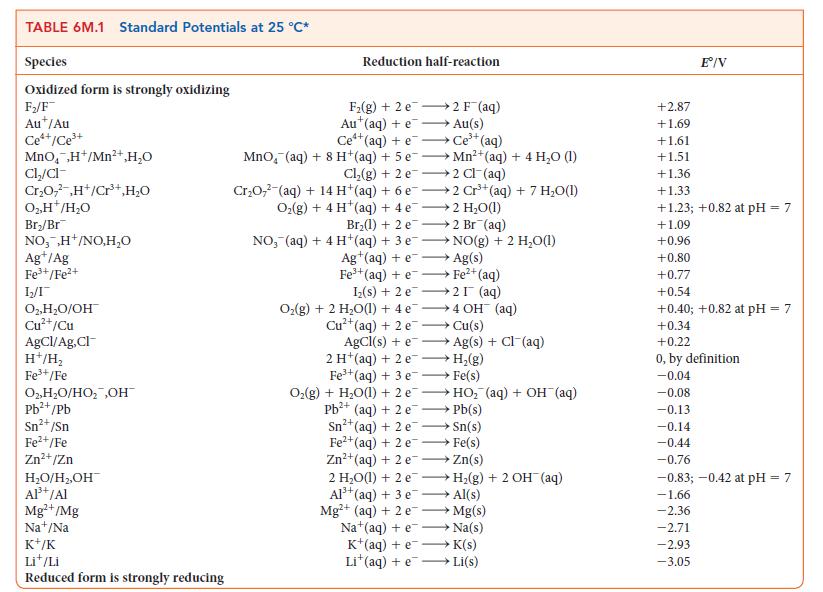
Transcribed Image Text:
TABLE 6M.1 Standard Potentials at 25 °C* Species Oxidized form is strongly oxidizing F₂/F Aut/Au Ce*+/Ce³+ MnO₂ ,H+/Mn²+,H₂O Cl₂/cl Cr₂O,²,H+/Cr³+,H₂O O₂, H*/H₂O Br₂/Br NO,,H+/NO,H₂O Ag+/Ag Fe³+/Fe²+ 1₂2/1 O2, H₂O/OH Cu²+/Cu AgCl/Ag,Cl- H+/H₂ Fe³+/Fe O₂, H₂O/HO₂ ,OH™ Pb²+/Pb Sn²+ /Sn Fe²+/Fe Zn²+/Zn H₂O/H₂,OH™ Al³+ /Al Mg²+/Mg Nat/Na K+/K Lit/Li Reduced form is strongly reducing Reduction half-reaction F₂(g) +2 e Aut(aq) +e Ce¹+(aq) +e Ce³+ (aq) MnO, (aq) + 8 H+(aq) + 5 eMn²+ (aq) + 4 H₂O (1) Cl₂(g) + 2e →2 Cl(aq) Cr₂O7² (aq) + 14 H+ (aq) + 6 e2 Cr³+ (aq) + 7 H₂O(1) Oz(g)+4H*(aq)+4e —+2H,O(l) 2 F¯ (aq) Au(s) Br₂(1) + 2 e2 Br (aq) NO, (aq) + 4H+(aq) + 3 e Ag+ (aq) + e Fe³+ (aq) +e→→→ Fe²+ (aq) AgCl (s) + e 2 H (aq) + 2 e Fe³+ (aq) + 3 e NO(g) + 2 H₂O(1) Ag(s) Iz(s) + 2 e 2 (aq) O₂(g) + 2 H₂O(1) + 4e4 OH (aq) →→→ Cu(s) 2+ Cu²+ (aq) + 2 e Fe²+(aq) + 2 e Zn²+ (aq) + 2 e →→→ Ag(s) + Cl¯(aq) H₂(g) Fe(s) O₂(g) + H₂O(1) + 2 eHO₂ (aq) + OH¯ (aq) Pb²+(aq) + 2e →→→→ Pb(s) Sn²+ (aq) + 2e →→→→Sn(s) →→→→Fe(s) Zn(s) 2 H₂O(l) + 2 eH₂(g) + 2 OH(aq) Al³+ (aq) + 3 e- →→→→ Al(s) Mg²+ (aq) + 2 e→→→→→→ Mg(s) Na (aq) + e→→→→→→→ Na(s) K+(aq) +e →→→ K(s) Lit(aq) +eLi(s) +2.87 +1.69 +1.61 +1.51 Eº/V +1.36 +1.33 +1.23; +0.82 at pH = 7 +1.09 +0.96 +0.80 +0.77 +0.54 +0.40; +0.82 at pH = 7 +0.34 +0.22 0, by definition -0.04 -0.08 -0.13 -0.14 -0.44 -0.76 -0.83; -0.42 at pH = 7 -1.66 -2.36 -2.71 -2.93 -3.05
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a 0...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Consider the question posed in Exercise 6N.19 except that a saturated calomel electrode (the solution is saturated with KCl instead of having [Cl ] = 1.00 mol L 1 ) is used in place of the standard...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
Smart housing Inc. is negotiating a deal to build a house. The owner wants to start in early spring when the weather begins to moderate and build through the summer into the fall. The completion time...
-
A plane, harmonic, acoustical wave that oscillates in air with an amplitude of 10-6 m has an intensity of 10-2 W/m2. What is the frequency of the sound wave?
-
Define each of the following terms: a. PV; i; INT; FVn; PVAn; FVAn; PMT; m; iNom b. FVIFi,n; PVIFi,n; FVIFAi,n; PVIFAi,n c. Opportunity cost rate d. Annuity; lump sum payment; cash flow; uneven cash...
-
7. Let a, b, c, D, E be real numbers with c =I- O. (a) If DE > 0, find all extrema of ax + by + cz subject to the constraint z = Dx2 + Ey2. Prove that a maximum occurs when cD < 0 and a minimum when...
-
The Piedmont School of Music has hired you as a consultant to help in analyzing the behavior of the schools costs. Use the account-classification method of cost estimation to classify each of the...
-
need urgent answers Q1: Explain the following with examples in context of financial accounting used in Sage50: a) Assets (Marks-5) b) Liabilities (Marks-5) c) Accrual concept (Marks-5) d) Trial...
-
Below are various transactions that took place during January for Anderson Corporation. Jan. 1: Anderson Company purchased merchandise inventory from Clarke Inc. for $25,000 with terms 2/10, n/30....
-
Calculate the molar concentration of OH in solutions with the following molar concentrations of H 3 O + : (a) 0.020 mol L 1 ; (b) 1.0 * 10 5 mol L 1 ; (c) 3.1 mol L 1 .
-
Below is the titration curve for the neutralization of 25 mL of a monoprotic acid with a strong base. Answer the following questions about the reaction and explain your reasoning in each case. (a) Is...
-
A thin, uniform 3.80-kg bar, 80.0 cm long, has very small 2.50-kg balls glued on at either end (Fig. 10.52). It is supported horizontally by a thin, horizontal, frictionless axle passing through its...
-
In the exchange lemma for the scheduling problem, we say that the first event to finish a* in a given time period [i,j] is always part of the optimal solution for that same time period. To argue...
-
4 10 points Company's year-end is December 31. Calculate depreciation for each year of the machine's estimated useful life under each of the following methods: (Do not round intermediate...
-
3. The walls of an oven are made from steel sheets with insulating board between them of thermal conductivity 0.18 J m-1 s -1 C-1 . If the maximum internal temperature in the oven is 300C and the...
-
Egyptian Spa produces two different spa products: Relax and Refresh. The company uses three operations to manufacture the products: mixing, blending, and packaging. Because of the materials used,...
-
Part A At a given instant A has the motion shown in (Figure 1). Determine the acceleration of B at this instant. Express your answer in feet per second squared to three significant figures. Enter...
-
For the cache design of the preceding problem, suppose that increasing the line size from one word to four words results in a decrease of the read miss rate from 3.2% to 1.1%. For both the non burst...
-
Teasdale Inc. manufactures and sells commercial and residential security equipment. The comparative unclassified balance sheets for December 31, 2015 and 2014 are provided below. Selected missing...
-
All of the following acid-base reactions are reactions that we will study in greater detail in the chapters to follow. For each one, draw the mechanism and then clearly label the acid, base,...
-
Each of the following mechanisms contains one or more errorsthat is, the curved arrows may or may not be correct. In each case, identify the errors and then describe what modification would be...
-
In an intramolecular proton transfer reaction, the acidic site and the basic site are tethered to the same molecule, and a proton is passed from the acidic region of the molecule to the basic region...
-
The following information is provided by Garden Gears for a new product it recently introduced: Total unit cost $50 Desired ROI per unit $22 Target selling price $72 How much is Garden Gears'...
-
Solid bank loan P5 million to a borrower on January 1, 2018. The terms of the loan require principal payments of P1 million each year for five years plus interest at 8%. The first principal and...
-
3) Assuming annual sales of $250,000 and a 50% gross (contribution) margin, calculate the following a. Average collection period if ending receivables total $45,000 b. Ending days-on-hand of...

Study smarter with the SolutionInn App


